Identification of an N-oxide pyridine GW4064 analog as a potent FXR agonist
Feng, S., Yang, M., Zhang, Z., Wang, Z., Hong, D., Richter, H., Benson, G.M., Bleicher, K., Grether, U., Martin, R.E., Plancher, J.-M., Kuhn, B., Rudolph, M.G., Chen, L.(2009) Bioorg Med Chem Lett 19: 2595-2598
- PubMed: 19328688
- DOI: https://doi.org/10.1016/j.bmcl.2009.03.008
- Primary Citation of Related Structures:
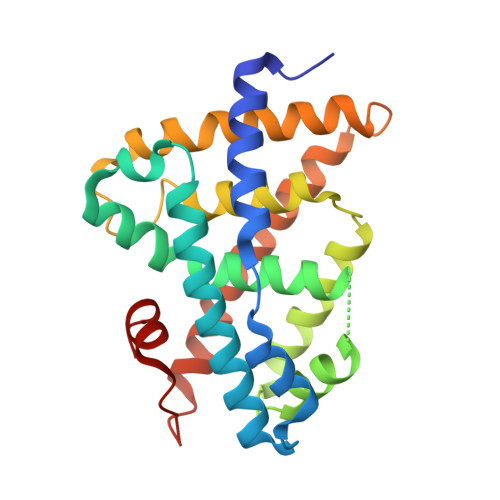
3FXV - PubMed Abstract:
According to the docking studies and the analysis of a co-crystal structure of GW4064 with FXR, a series of 3-aryl heterocyclic isoxazole analogs were designed and synthesized. N-Oxide pyridine analog (7b) was identified as a promising FXR agonist with potent binding affinity and good efficacy, supporting our hypothesis that through an additional hydrogen bond interaction between the pyridine substituent of isoxazole analogs and Tyr373 and Ser336 of FXR, binding affinity and functional activity could be improved.
Organizational Affiliation:
Roche R&D Center(China) Ltd, 720 Cai Lun Road, Building 5, Pudong, Shanghai 201203, China.