Type I and type II fatty acid biosynthesis in Eimeria tenella: Enoyl reductase activity and structure
Lu, J.Z., Muench, S.P., Allary, M., Campbell, S., Roberts, C.W., Mui, E., McLeod, R.L., Rice, D.W., Prigge, S.T.(2007) Parasitology 134: 1949-1962
- PubMed: 17697396
- DOI: https://doi.org/10.1017/S0031182007003319
- Primary Citation of Related Structures:
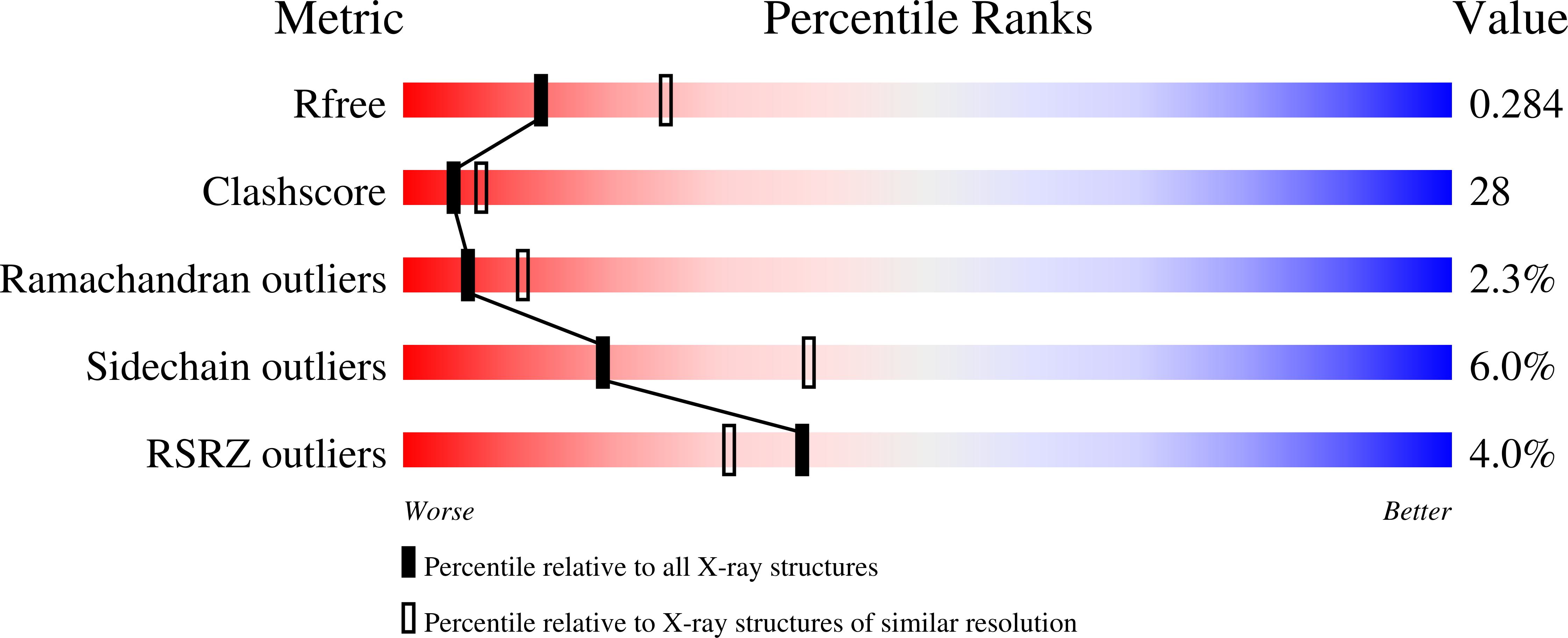
2PTG - PubMed Abstract:
Apicomplexan parasites of the genus Eimeria are the major causative agent of avian coccidiosis, leading to high economic losses in the poultry industry. Recent results show that Eimeria tenella harbours an apicoplast organelle, and that a key biosynthetic enzyme, enoyl reductase, is located in this organelle. In related parasites, enoyl reductase is one component of a type II fatty acid synthase (FAS) and has proven to be an attractive target for antimicrobial compounds. We cloned and expressed the mature form of E. tenella enoyl reductase (EtENR) for biochemical and structural studies. Recombinant EtENR exhibits NADH-dependent enoyl reductase activity and is inhibited by triclosan with an IC50 value of 60 nm. The crystal structure of EtENR reveals overall similarity with other ENR enzymes; however, the active site of EtENR is unoccupied, a state rarely observed in other ENR structures. Furthermore, the position of the central beta-sheet appears to block NADH binding and would require significant movement to allow NADH binding, a feature not previously seen in the ENR family. We analysed the E. tenella genomic database for orthologues of well-characterized bacterial and apicomplexan FAS enzymes and identified 6 additional genes, suggesting that E. tenella contains a type II FAS capable of synthesizing saturated, but not unsaturated, fatty acids. Interestingly, we also identified sequences that appear to encode multifunctional type I FAS enzymes, a feature also observed in Toxoplasma gondii, highlighting the similarity between these apicomplexan parasites.
Organizational Affiliation:
Department of Molecular Microbiology & Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.