Conserved region I of human coactivator TAF4 binds to a short hydrophobic motif present in transcriptional regulators.
Wang, X., Truckses, D.M., Takada, S., Matsumura, T., Tanese, N., Jacobson, R.H.(2007) Proc Natl Acad Sci U S A 104: 7839-7844
- PubMed: 17483474
- DOI: https://doi.org/10.1073/pnas.0608570104
- Primary Citation of Related Structures:
2P6V - PubMed Abstract:
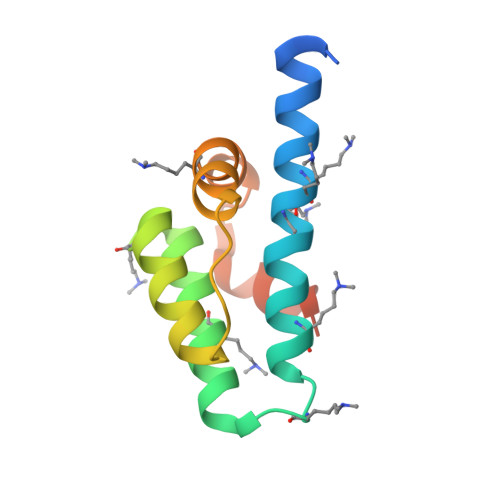
TBP-associated factor 4 (TAF4), an essential subunit of the TFIID complex acts as a coactivator for multiple transcriptional regulators, including Sp1 and CREB. However, little is known regarding the structural properties of the TAF4 subunit that lead to the coactivator function. Here, we report the crystal structure at 2.0-A resolution of the human TAF4-TAFH domain, a conserved domain among all metazoan TAF4, TAF4b, and ETO family members. The hTAF4-TAFH structure adopts a completely helical fold with a large hydrophobic groove that forms a binding surface for TAF4 interacting factors. Using peptide phage display, we have characterized the binding preference of the hTAF4-TAFH domain for a hydrophobic motif, DPsiPsizetazetaPsiPhi, that is present in a number of nuclear factors, including several important transcriptional regulators with roles in activating, repressing, and modulating posttranslational modifications. A comparison of the hTAF4-TAFH structure with the homologous ETO-TAFH domain reveals several critical residues important for hTAF4-TAFH target specificity and suggests that TAF4 has evolved in response to the increased transcriptional complexity of metazoans.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Graduate School in Biomedical Sciences Program in Genes and Development, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.