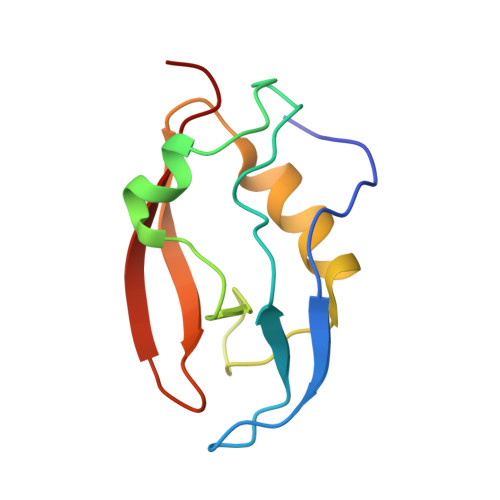
Solution structure of Q388A3 PDZ domain from Trypanosoma brucei
Mei, S., Dong, Y., Zhang, J., Zhang, X., Tu, X.(2016) J Struct Biol 194: 214-217
- PubMed: 26917351
- DOI: https://doi.org/10.1016/j.jsb.2016.02.018
- Primary Citation of Related Structures:
2NB4 - PubMed Abstract:
PDZ domains are abundant protein interaction modules that often recognize short amino acid motifs at the C-termini of target proteins and regulate multiple biological processes. So far, no PDZ domain in Trypanosoma brucei, an eukaryotic parasite causing sleeping sickness, has been studied. Q388A3, conserved in the related kinetoplastid parasites, is a 1634-residue protein containing a PDZ domain at its C-terminus. In this work, the solution structure of Q388A3 PDZ domain was solved by NMR spectroscopy. Q388A3 PDZ domain adopts a PDZ-like fold composed by a five-stranded β-sheet capped by two α-helices, which is similar to the PDZ domains from HtrA family proteins. Meanwhile, Q388A3 PDZ domain shows some structural features quite different from HtrA PDZ domain.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale, School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230026, PR China.