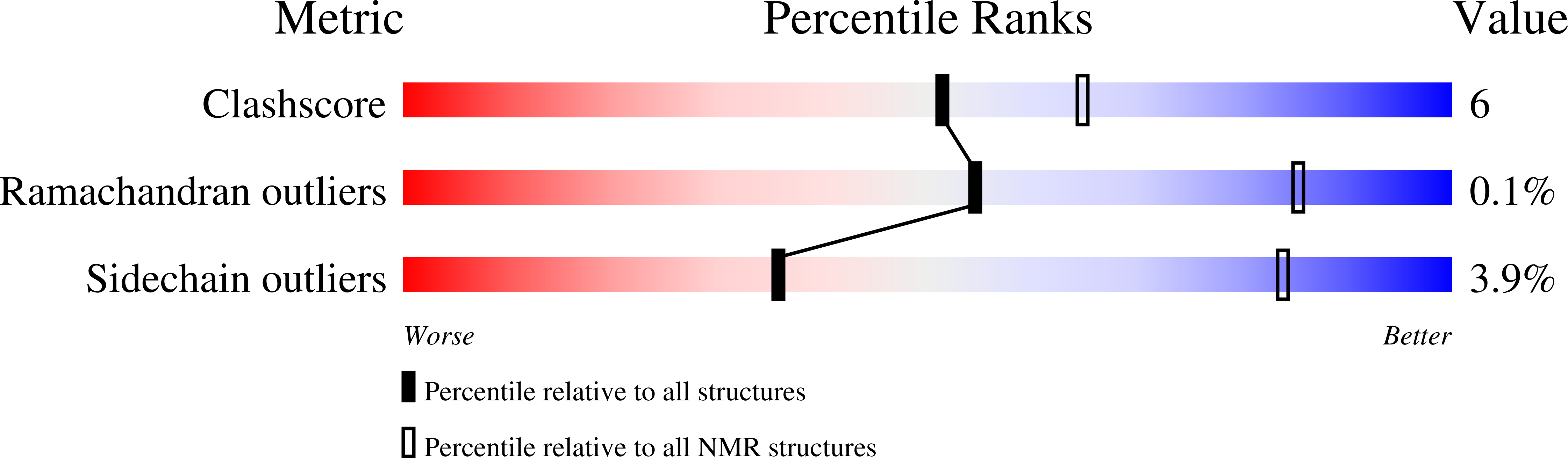Structure-function analysis reveals that the Pseudomonas aeruginosa Tps4 two-partner secretion system is involved in CupB5 translocation.
Garnett, J.A., Muhl, D., Douse, C.H., Hui, K., Busch, A., Omisore, A., Yang, Y., Simpson, P., Marchant, J., Waksman, G., Matthews, S., Filloux, A.(2015) Protein Sci 24: 670-687
- PubMed: 25641651
- DOI: https://doi.org/10.1002/pro.2640
- Primary Citation of Related Structures:
2MHJ - PubMed Abstract:
Pseudomonas aeruginosa is a Gram-negative opportunistic bacterium, synonymous with cystic fibrosis patients, which can cause chronic infection of the lungs. This pathogen is a model organism to study biofilms: a bacterial population embedded in an extracellular matrix that provide protection from environmental pressures and lead to persistence. A number of Chaperone-Usher Pathways, namely CupA-CupE, play key roles in these processes by assembling adhesive pili on the bacterial surface. One of these, encoded by the cupB operon, is unique as it contains a nonchaperone-usher gene product, CupB5. Two-partner secretion (TPS) systems are comprised of a C-terminal integral membrane β-barrel pore with tandem N-terminal POTRA (POlypeptide TRansport Associated) domains located in the periplasm (TpsB) and a secreted substrate (TpsA). Using NMR we show that TpsB4 (LepB) interacts with CupB5 and its predicted cognate partner TpsA4 (LepA), an extracellular protease. Moreover, using cellular studies we confirm that TpsB4 can translocate CupB5 across the P. aeruginosa outer membrane, which contrasts a previous observation that suggested the CupB3 P-usher secretes CupB5. In support of our findings we also demonstrate that tps4/cupB operons are coregulated by the RocS1 sensor suggesting P. aeruginosa has developed synergy between these systems. Furthermore, we have determined the solution-structure of the TpsB4-POTRA1 domain and together with restraints from NMR chemical shift mapping and in vivo mutational analysis we have calculated models for the entire TpsB4 periplasmic region in complex with both TpsA4 and CupB5 secretion motifs. The data highlight specific residues for TpsA4/CupB5 recognition by TpsB4 in the periplasm and suggest distinct roles for each POTRA domain.
Organizational Affiliation:
Department of Life Sciences, Centre for Structural Biology, Imperial College London, South Kensington Campus, London, SW7 2AZ, United Kingdom; Department of Life Sciences, MRC Centre for Molecular Bacteriology and Infection, Imperial College London, South Kensington Campus, London, SW7 2AZ, United Kingdom.