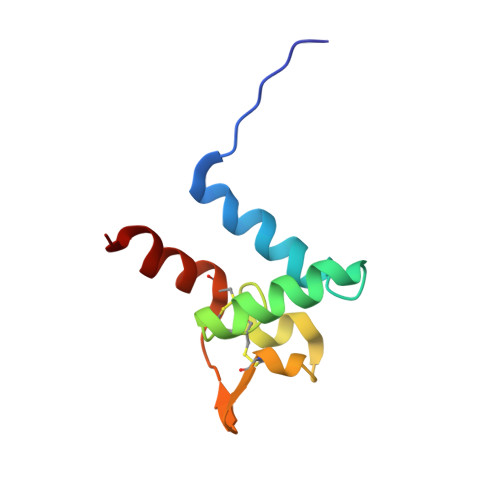
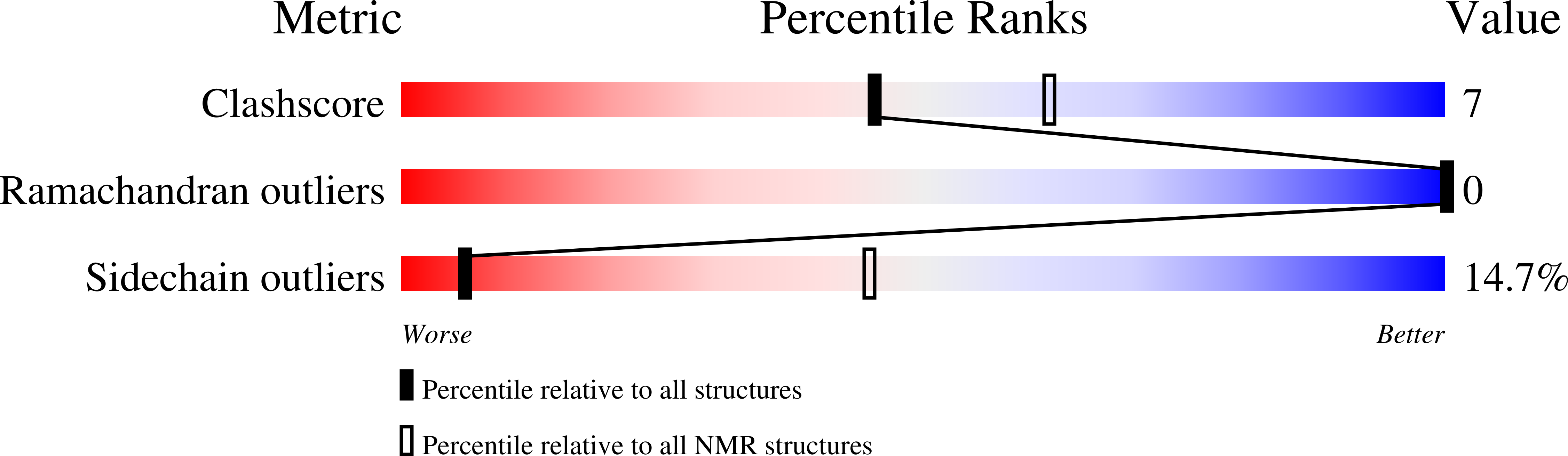Solution structure, membrane interactions, and protein binding partners of the tetraspanin Sm-TSP-2, a vaccine antigen from the human blood fluke Schistosoma mansoni
Jia, X., Schulte, L., Loukas, A., Pickering, D., Pearson, M., Mobli, M., Jones, A., Rosengren, K.J., Daly, N.L., Gobert, G.N., Jones, M.K., Craik, D.J., Mulvenna, J.(2014) J Biol Chem 289: 7151-7163
- PubMed: 24429291
- DOI: https://doi.org/10.1074/jbc.M113.531558
- Primary Citation of Related Structures:
2M7Z - PubMed Abstract:
The tetraspanins (TSPs) are a family of integral membrane proteins that are ubiquitously expressed at the surface of eukaryotic cells. TSPs mediate a range of processes at the surface of the plasma membrane by providing a scaffold for the assembly of protein complexes known as tetraspanin-enriched microdomains (TEMs). We report here the structure of the surface-exposed EC2 domain from Sm-TSP-2, a TSP from Schistosoma mansoni and one of the better prospects for the development of a vaccine against schistosomiasis. This is the first solution structure of this domain, and our investigations of its interactions with lipid micelles provide a general model for interactions between TSPs, membranes, and other proteins. Using chemical cross-linking, eight potential protein constituents of Sm-TSP-2-mediated TEMs were also identified. These include proteins important for membrane maintenance and repair, providing further evidence for the functional role of Sm-TSP-2- and Sm-TSP-2-mediated TEMs. The identification of calpain, Sm29, and fructose-bisphosphate aldolase, themselves potential vaccine antigens, suggests that the Sm-TSP-2-mediated TEMs could be disrupted via multiple targets. The identification of further Sm-TSP-2-mediated TEM proteins increases the available candidates for multiplex vaccines and/or novel drugs targeting TEMs in the schistosome tegument.
Organizational Affiliation:
Queensland Institute of Medical Research, Brisbane, QLD 4006, Australia.