Structures and interaction analyses of the integrin alphaMbeta2 cytoplasmic tails
Chua, G.L., Tang, X.Y., Amalraj, M., Tan, S.M., Bhattacharjya, S.(2011) J Biol Chem
- PubMed: 22052909
- DOI: https://doi.org/10.1074/jbc.M111.280164
- Primary Citation of Related Structures:
2LKE, 2LKJ - PubMed Abstract:
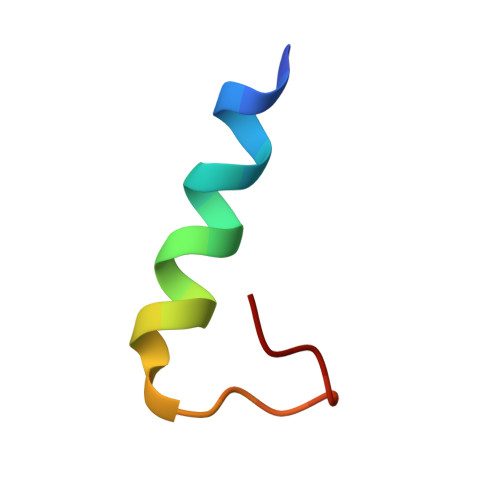
Integrins are heterodimeric (α and β subunits) signal transducer proteins involved in cell adhesions and migrations. The cytosolic tails of integrins are essential for transmitting bidirectional signaling and also implicated in maintaining the resting states of the receptors. In addition, cytosolic tails of integrins often undergo post-translation modifications like phosphorylation. However, the consequences of phosphorylation on the structures and interactions are not clear. The leukocyte-specific integrin αMβ2 is essential for myeloid cell adhesion, phagocytosis, and degranulation. In this work, we determined solution structures of the myristoylated cytosolic tail of αM and a Ser phosphorylated variant in dodecylphosphocholine micelles by NMR spectroscopy. Furthermore, the interactions between non-phosphorylated and phosphorylated αM tails with β2 tail were investigated by NMR and fluorescence resonance energy transfer (FRET). The three-dimensional structures of the 24-residue cytosolic tail of αM or phosphorylated αM are characterized by an N-terminal amphipathic helix and a loop at the C terminus. The residues at the loop are involved in packing interactions with the hydrophobic face of the helix. 15N-1H heteronuclear single quantum coherence experiments identified residues of αM and β2 tails that may be involved in the formation of a tail-tail heterocomplex. We further examined interactions between myristoylated β2 tail in dodecylphosphocholine micelles with dansylated αM tail peptides by FRET. These studies revealed enhanced interactions between αM or phosphorylated αM tails with β2 tail with Kd values ∼5.2±0.6 and ∼4.4±0.7 μm, respectively. Docked structures of tail-tail complexes delineated that the αM/β2 interface at the cytosolic region could be sustained by a network of polar interactions, ionic interactions, and/or hydrogen bonds.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore.