Structural Insight into Unique Cardiac Myosin-binding Protein-C Motif: A PARTIALLY FOLDED DOMAIN.
Howarth, J.W., Ramisetti, S., Nolan, K., Sadayappan, S., Rosevear, P.R.(2012) J Biol Chem 287: 8254-8262
- PubMed: 22235120
- DOI: https://doi.org/10.1074/jbc.M111.309591
- Primary Citation of Related Structures:
2LHU - PubMed Abstract:
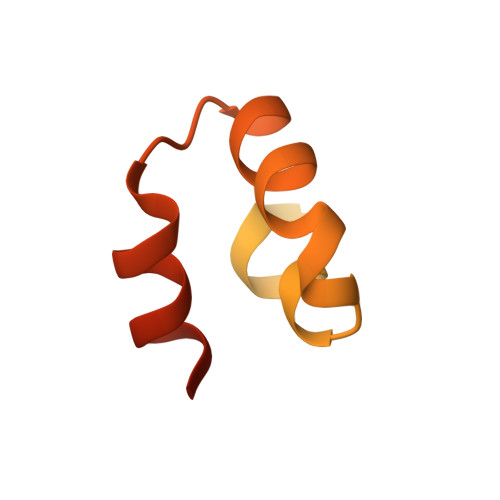
The structural role of the unique myosin-binding motif (m-domain) of cardiac myosin-binding protein-C remains unclear. Functionally, the m-domain is thought to directly interact with myosin, whereas phosphorylation of the m-domain has been shown to modulate interactions between myosin and actin. Here we utilized NMR to analyze the structure and dynamics of the m-domain in solution. Our studies reveal that the m-domain is composed of two subdomains, a largely disordered N-terminal portion containing three known phosphorylation sites and a more ordered and folded C-terminal portion. Chemical shift analyses, d(NN)(i, i + 1) NOEs, and (15)N{(1)H} heteronuclear NOE values show that the C-terminal subdomain (residues 315-351) is structured with three well defined helices spanning residues 317-322, 327-335, and 341-348. The tertiary structure was calculated with CS-Rosetta using complete (13)C(α), (13)C(β), (13)C', (15)N, (1)H(α), and (1)H(N) chemical shifts. An ensemble of 20 acceptable structures was selected to represent the C-terminal subdomain that exhibits a novel three-helix bundle fold. The solvent-exposed face of the third helix was found to contain the basic actin-binding motif LK(R/K)XK. In contrast, (15)N{(1)H} heteronuclear NOE values for the N-terminal subdomain are consistent with a more conformationally flexible region. Secondary structure propensity scores indicate two transient helices spanning residues 265-268 and 293-295. The presence of both transient helices is supported by weak sequential d(NN)(i, i + 1) NOEs. Thus, the m-domain consists of an N-terminal subdomain that is flexible and largely disordered and a C-terminal subdomain having a three-helix bundle fold, potentially providing an actin-binding platform.
Organizational Affiliation:
Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati College of Medicine, Cincinnati, Ohio 45267, USA.