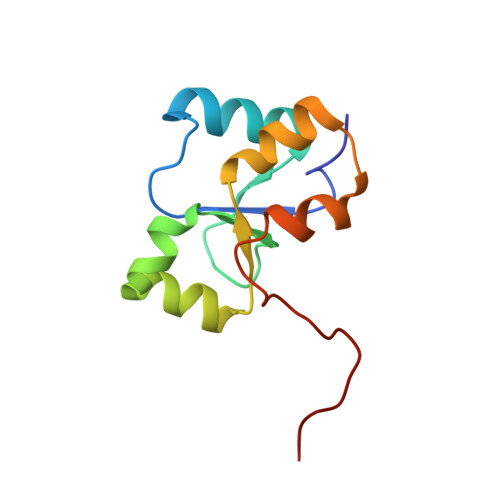
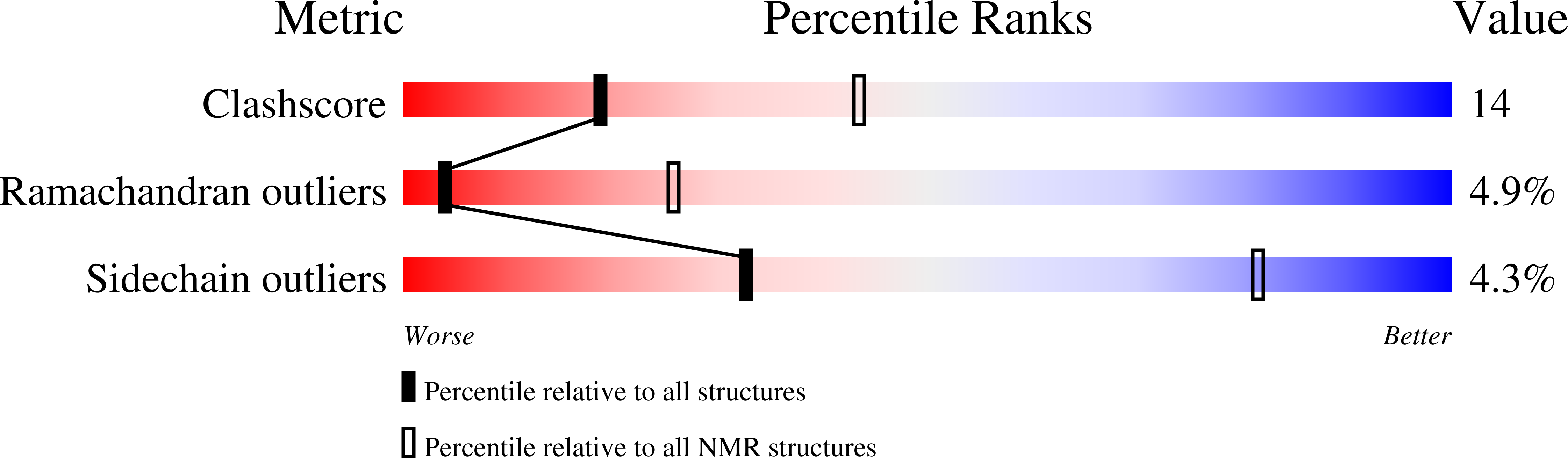Structural studies of the PARP-1 BRCT domain.
Loeffler, P.A., Cuneo, M.J., Mueller, G.A., Derose, E.F., Gabel, S.A., London, R.E.(2011) BMC Struct Biol 11: 37-37
- PubMed: 21967661
- DOI: https://doi.org/10.1186/1472-6807-11-37
- Primary Citation of Related Structures:
2LE0 - PubMed Abstract:
Poly(ADP-ribose) polymerase-1 (PARP-1) is one of the first proteins localized to foci of DNA damage. Upon activation by encountering nicked DNA, the PARP-1 mediated trans-poly(ADP-ribosyl)ation of DNA binding proteins occurs, facilitating access and accumulation of DNA repair factors. PARP-1 also auto-(ADP-ribosyl)ates its central BRCT-containing domain forming part of an interaction site for the DNA repair scaffolding protein X-ray cross complementing group 1 protein (XRCC1). The co-localization of XRCC1, as well as bound DNA repair factors, to sites of DNA damage is important for cell survival and genomic integrity.
Organizational Affiliation:
Department of Chemistry, Sam Houston State University, Huntsville, Texas 77340, USA.