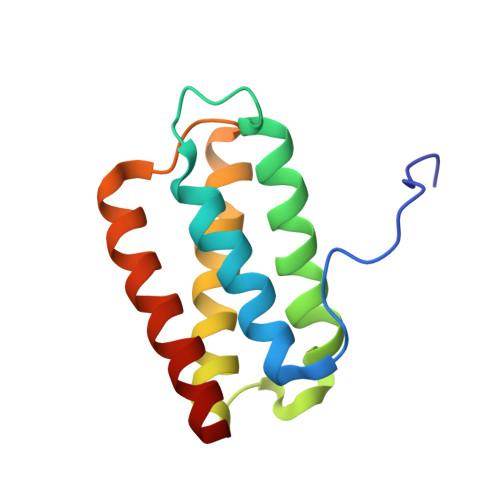
Solution structure of Psb27 from cyanobacterial photosystem II
Mabbitt, P.D., Rautureau, G.J., Day, C.L., Wilbanks, S.M., Eaton-Rye, J.J., Hinds, M.G.(2009) Biochemistry 48: 8771-8773
- PubMed: 19697958
- DOI: https://doi.org/10.1021/bi901309c
- Primary Citation of Related Structures:
2KMF - PubMed Abstract:
Psb27 is a highly conserved component of photosystem II. The three-dimensional structure has a well-defined helical core, composed of four helices arranged in a right-handed up-down-up-down fold, with a less ordered region of the structure located at the N-terminus. The position of conserved residues on the surface suggests conserved functional roles for distinct interconnected features encompassing a P-phi-P loop, a polar patch spanning helices alpha3 and alpha4, and the N-terminal sequence.
Organizational Affiliation:
Department of Biochemistry, University of Otago, Dunedin 9054, New Zealand.