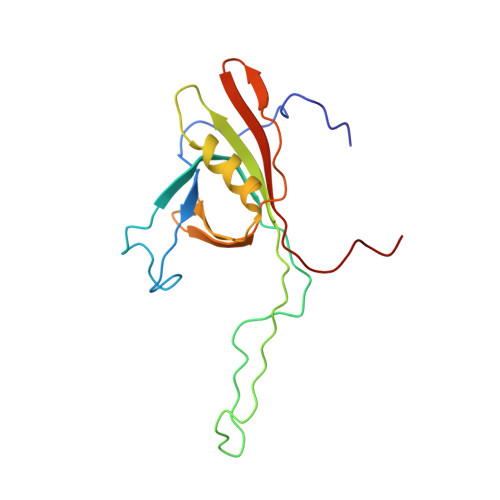
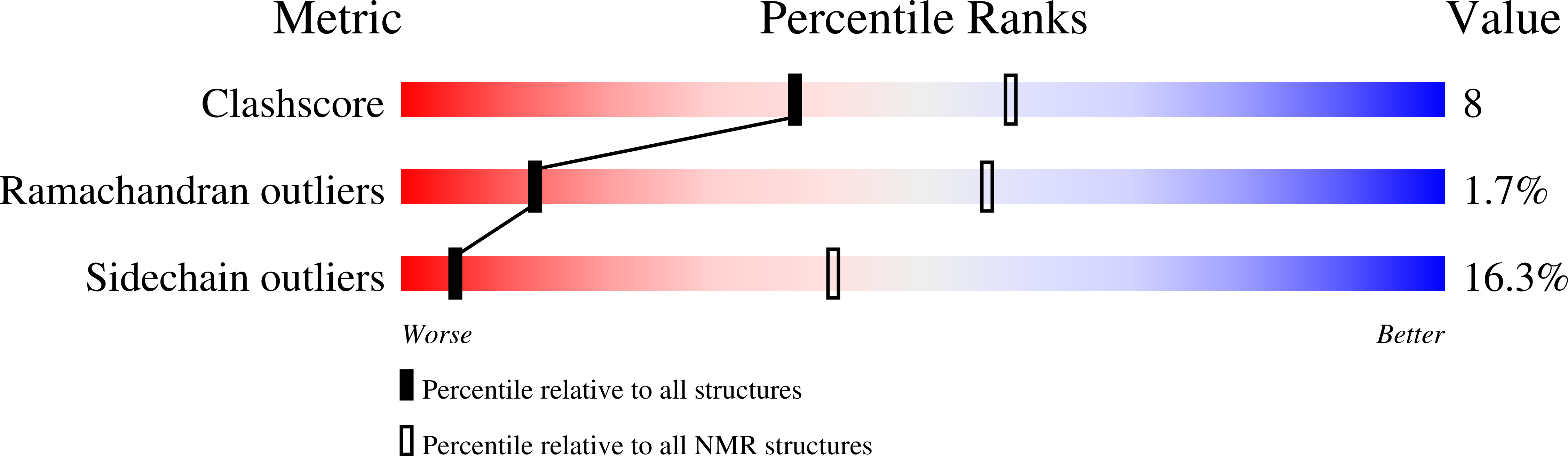The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system.
Pell, L.G., Kanelis, V., Donaldson, L.W., Howell, P.L., Davidson, A.R.(2009) Proc Natl Acad Sci U S A 106: 4160-4165
- PubMed: 19251647
- DOI: https://doi.org/10.1073/pnas.0900044106
- Primary Citation of Related Structures:
2K4Q - PubMed Abstract:
Most bacteriophages possess long tails, which serve as the conduit for genome delivery. We report the solution structure of the N-terminal domain of gpV, the protein comprising the major portion of the noncontractile phage lambda tail tube. This structure is very similar to a previously solved tail tube protein from a contractile-tailed phage, providing the first direct evidence of an evolutionary connection between these 2 distinct types of phage tails. A remarkable structural similarity is also seen to Hcp1, a component of the bacterial type VI secretion system. The hexameric structure of Hcp1 and its ability to form long tubes are strikingly reminiscent of gpV when it is polymerized into a tail tube. These data coupled with other similarities between phage and type VI secretion proteins support an evolutionary relationship between these systems. Using Hcp1 as a model, we propose a polymerization mechanism for gpV involving several disorder-to-order transitions.
Organizational Affiliation:
Department of Biochemistry, University of Toronto, Medical Sciences Building, Toronto, ON M5S 1A8, Canada.