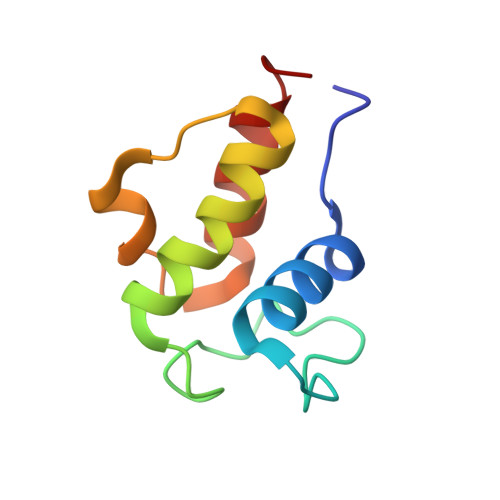
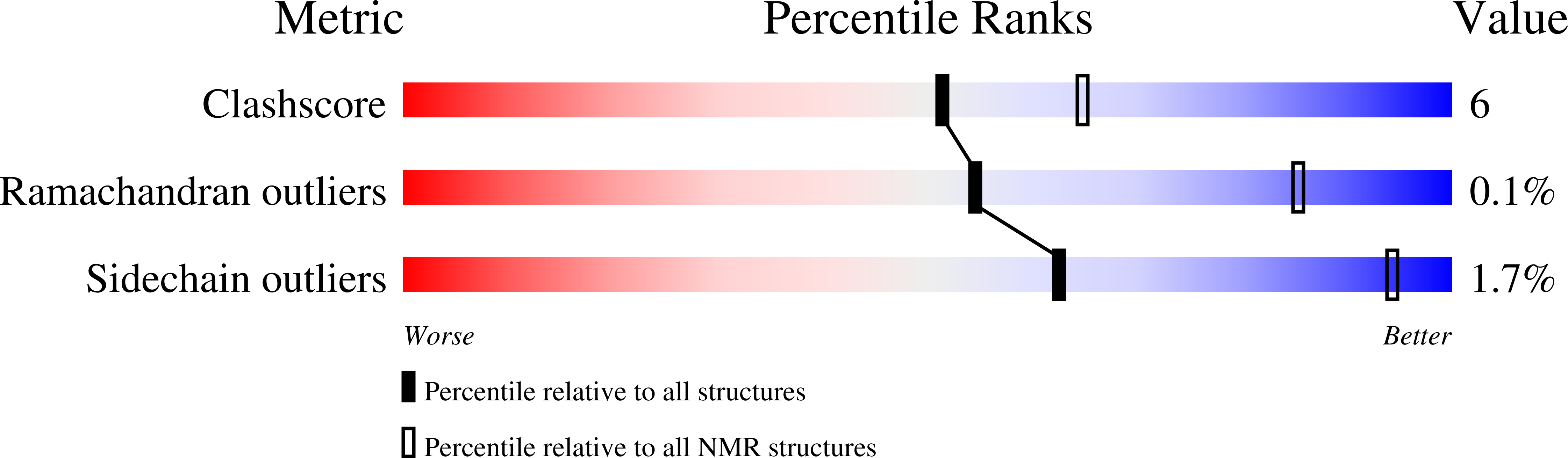An ACP Structural Switch: Conformational Differences between the Apo and Holo Forms of the Actinorhodin Polyketide Synthase Acyl Carrier Protein.
Evans, S.E., Williams, C., Arthur, C.J., Burston, S.G., Simpson, T.J., Crosby, J., Crump, M.P.(2008) Chembiochem 9: 2424-2432
- PubMed: 18770515
- DOI: https://doi.org/10.1002/cbic.200800180
- Primary Citation of Related Structures:
2K0X, 2K0Y - PubMed Abstract:
The actinorhodin (act) synthase acyl carrier protein (ACP) from Streptomyces coelicolor plays a central role in polyketide biosynthesis. Polyketide intermediates are bound to the free sulfhydryl group of a phosphopantetheine arm that is covalently linked to a conserved serine residue in the holo form of the ACP. The solution NMR structures of both the apo and holo forms of the ACP are reported, which represents the first high resolution comparison of these two forms of an ACP. Ensembles of twenty apo and holo structures were calculated and yielded atomic root mean square deviations of well-ordered backbone atoms to the average coordinates of 0.37 and 0.42 A, respectively. Three restraints defining the protein to the phosphopantetheine interface were identified. Comparison of the apo and holo forms revealed previously undetected conformational changes. Helix III moved towards helix II (contraction of the ACP), and Leu43 on helix II subtly switched from being solvent exposed to forming intramolecular interactions with the newly added phosphopantetheine side chain. Tryptophan fluorescence and S. coelicolor fatty acid synthase (FAS) holo-synthase (ACPS) assays indicated that apo-ACP has a twofold higher affinity (K(d) of 1.1 muM) than holo-ACP (K(d) of 2.1 muM) for ACPS. Site-directed mutagenesis of Leu43 and Asp62 revealed that both mutations affect binding, but have differential affects on modification by ACPS. Leu43 mutations in particular strongly modulate binding affinity for ACPS. Comparison of apo- and holo-ACP structures with known models of the Bacillus subtilis FAS ACP-holo-acyl carrier protein synthase (ACPS) complex suggests that conformational modulation of helix II and III between apo- and holo-ACP could play a role in dissociation of the ACP-ACPS complex.
Organizational Affiliation:
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK), Fax: (+44) 117-9298611.