A Twin Set of Low Pka Arginines Ensures the Concerted Acid Base Catalytic Mechanism of Cyanase
Guilloton, M., Walsh, M.A., Joachimiak, A., Anderson, P.M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| CYANATE LYASE | 156 | Escherichia coli | Mutation(s): 1 EC: 4.2.1.104 | 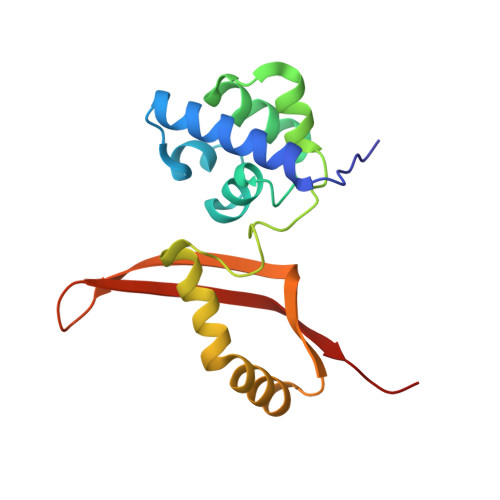 | |
UniProt | |||||
Find proteins for P00816 (Escherichia coli (strain K12)) Explore P00816 Go to UniProtKB: P00816 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00816 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | BA [auth E] CA [auth E] DB [auth J] EB [auth J] FA [auth F] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| AZI Query on AZI | R [auth C] | AZIDE ION N3 IVRMZWNICZWHMI-UHFFFAOYSA-N |  | ||
| CL Query on CL | AA [auth E] AB [auth I] BB [auth J] CB [auth J] DA [auth E] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.392 | α = 70.69 |
| b = 80.897 | β = 75.98 |
| c = 81.094 | γ = 65.15 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |