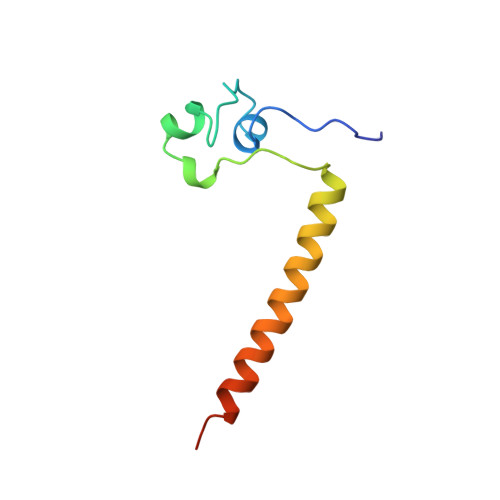
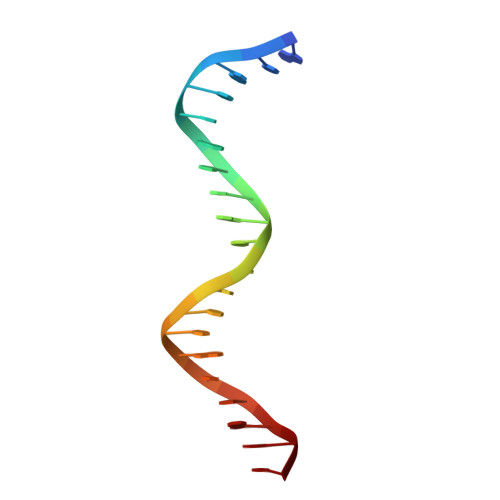
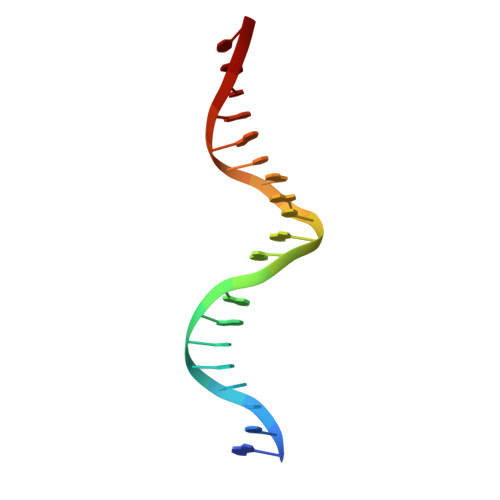
Structure of HAP1-18-DNA implicates direct allosteric effect of protein-DNA interactions on transcriptional activation.
King, D.A., Zhang, L., Guarente, L., Marmorstein, R.(1999) Nat Struct Biol 6: 22-27
- PubMed: 9886287
- DOI: https://doi.org/10.1038/4893
- Primary Citation of Related Structures:
2HAP - PubMed Abstract:
HAP1 is a yeast transcriptional activator that binds with equal affinity to the dissimilar upstream activation sequences UAS1 and UAS(CYC7), but activates transcription differentially when bound to each site. HAP1-18 harbors an amino acid change in the DNA binding domain. While binding UAS1 poorly, HAP1-18 binds UAS(CYC7) with wild-type properties and activates transcription at elevated levels relative to HAP1. We have determined the structure of HAP1-18-UAS(CYC7) and have compared it to HAP1-UAS(CYC7). Unexpectedly, the single amino acid substitution in HAP1-18 nucleates a significantly altered hydrogen bond interface between the protein and DNA resulting in DNA conformational changes and an ordering of one N-terminal arm of the protein dimer along the DNA minor groove. These observations, together with a large subset of transcriptionally defective mutations in the HAP1 DNA-binding domain that map to the HAP1-DNA interface, suggest that protein-DNA interactions may have direct allosteric effects on transcriptional activation.
- The Wistar Institute and The Department of Chemistry, University of Pennsylvania, Philadelphia 19104, USA.
Organizational Affiliation: