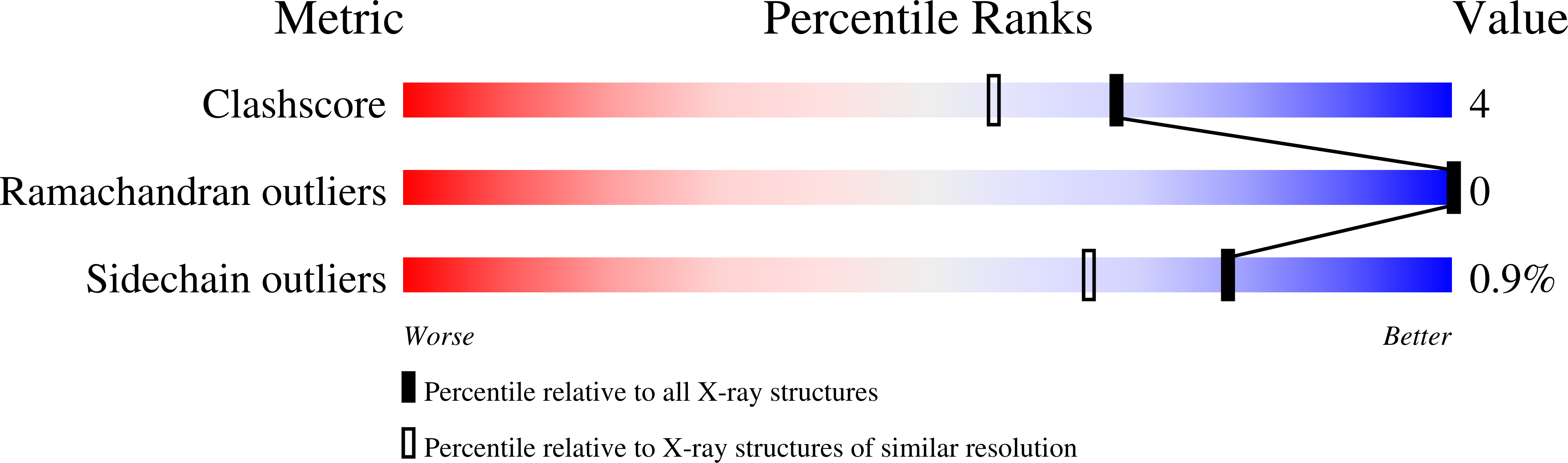Weak Activity of Haloalkane Dehalogenase Linb with 1,2,3-Trichloropropane Revealed by X-Ray Crystallography and Microcalorimetry
Minincova, M., Prokop, Z., Vevodova, J., Nagata, Y., Damborsky, J.(2007) Appl Environ Microbiol 73: 2005
- PubMed: 17259360
- DOI: https://doi.org/10.1128/AEM.02416-06
- Primary Citation of Related Structures:
2BFN - PubMed Abstract:
1,2,3-Trichloropropane (TCP) is a highly toxic and recalcitrant compound. Haloalkane dehalogenases are bacterial enzymes that catalyze the cleavage of a carbon-halogen bond in a wide range of organic halogenated compounds. Haloalkane dehalogenase LinB from Sphingobium japonicum UT26 has, for a long time, been considered inactive with TCP, since the reaction cannot be easily detected by conventional analytical methods. Here we demonstrate detection of the weak activity (k(cat) = 0.005 s(-1)) of LinB with TCP using X-ray crystallography and microcalorimetry. This observation makes LinB a useful starting material for the development of a new biocatalyst toward TCP by protein engineering. Microcalorimetry is proposed to be a universal method for the detection of weak enzymatic activities. Detection of these activities is becoming increasingly important for engineering novel biocatalysts using the scaffolds of proteins with promiscuous activities.
Organizational Affiliation:
Loschmidt Laboratories, Masaryk University, Kamenice 5/A4, 625 00 Brno, Czech Republic.