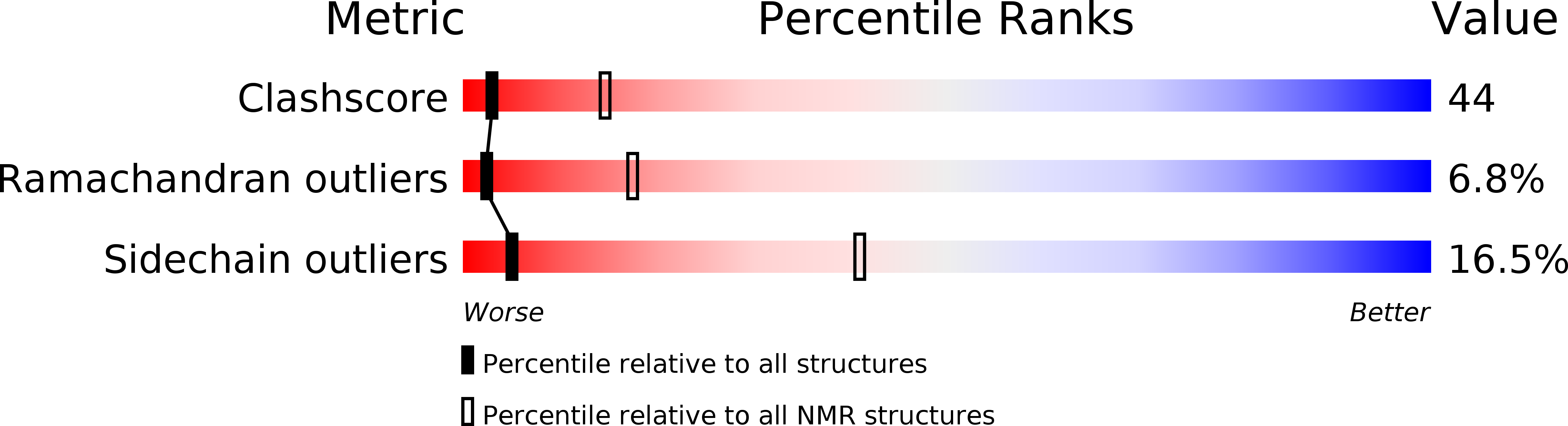Solution structure of NOD1 CARD and mutational analysis of its interaction with the CARD of downstream kinase RICK.
Manon, F., Favier, A., Nunez, G., Simorre, J.P., Cusack, S.(2007) J Mol Biol 365: 160-174
- PubMed: 17054981
- DOI: https://doi.org/10.1016/j.jmb.2006.09.067
- Primary Citation of Related Structures:
2B1W - PubMed Abstract:
NOD1 is a cytosolic signalling host pattern-recognition receptor composed of a caspase-activating and recruitment domain (CARD), a nucleotide-binding and oligomerization domain (NOD) and leucine-rich repeats. It plays a crucial role in innate immunity by activating the NF-kappaB pathway via its downstream effector the kinase RICK (RIP2) following the recognition of a specific bacterial ligand. RICK is recruited by NOD1 through interaction of their respective CARDs. Here we present the high resolution NMR structure of the NOD1 CARD. It is generally similar to other CARDs of known structure, consisting of six tightly packed helices, although the length and orientation of the last helix is unusual. Mutations in both the NOD1 and RICK CARD domains were assayed by immuno-precipitation of cell lysates and in vivo NF-kappaB activation in order to define residues important for CARD-CARD interaction and downstream signalling. The results show that the interaction is critically dependent on three acidic residues on NOD1 CARD and three basic residues on RICK CARD and thus is likely to have a strong electrostatic component, similar to other characterised CARD-CARD interactions.
Organizational Affiliation:
Grenoble Outstation, European Molecular Biology Laboratory, 6 rue Jules Horowitz, BP 181, F-38042 Grenoble Cedex 9, France.