Methanoarchaeal Sulfolactate Dehydrogenase: Prototype of a New Family of Nadh-Dependent Enzymes.
Irimia, A., Madern, D., Vellieux, F.M.D.(2004) EMBO J 23: 1234
- PubMed: 15014443
- DOI: https://doi.org/10.1038/sj.emboj.7600147
- Primary Citation of Related Structures:
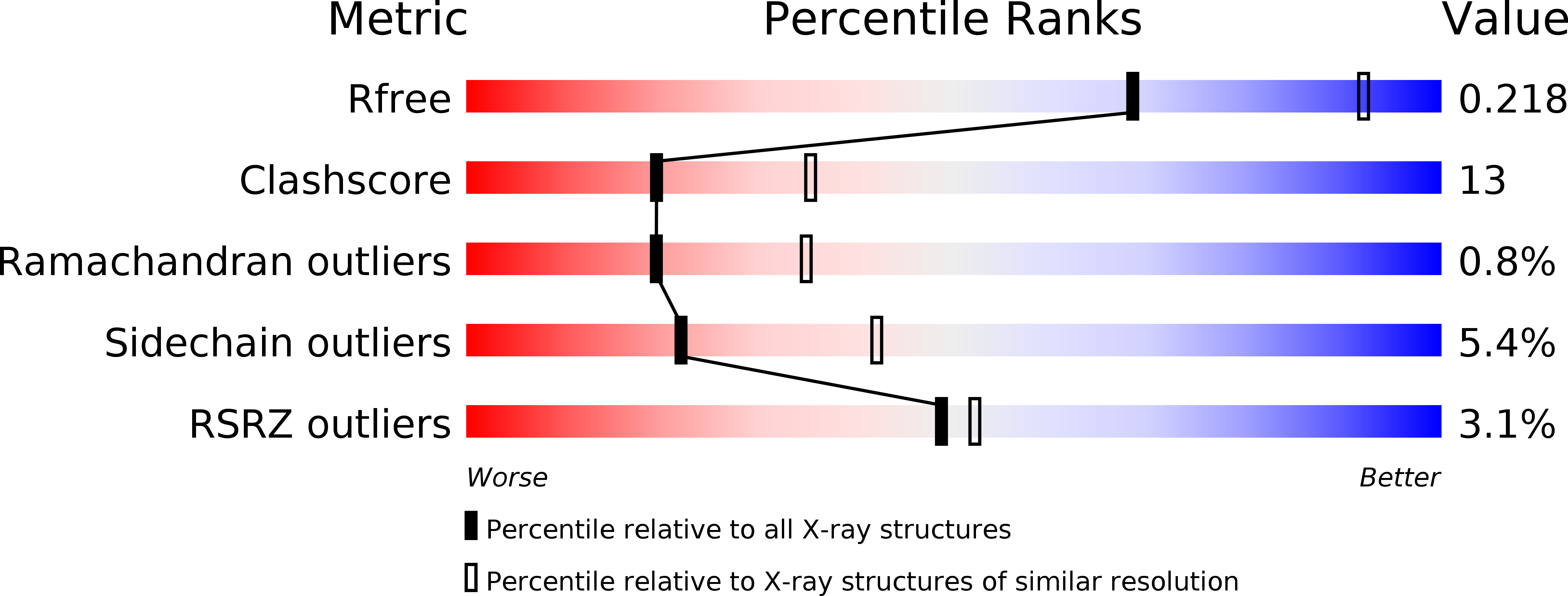
2X06 - PubMed Abstract:
The crystal structure of the sulfolactate dehydrogenase from the hyperthermophilic and methanogenic archaeon Methanocaldococcus jannaschii was solved at 2.5 A resolution (PDB id. 1RFM). The asymmetric unit contains a tetramer of tight dimers. This structure, complexed with NADH, does not contain a cofactor-binding domain with 'Rossmann-fold' topology. Instead, the tertiary and quaternary structures indicate a novel fold. The NADH is bound in an extended conformation in each active site, in a manner that explains the pro-S specificity. Cofactor binding involves residues belonging to both subunits within the tight dimers, which are therefore the smallest enzymatically active units. The protein was found to be a homodimer in solution by size-exclusion chromatography, analytical ultracentrifugation and small-angle neutron scattering. Various compounds were tested as putative substrates. The results indicate the existence of a substrate discrimination mechanism, which involves electrostatic interactions. Based on sequence homology and phylogenetic analyses, several other enzymes were classified as belonging to this novel family of homologous (S)-2-hydroxyacid dehydrogenases.
Organizational Affiliation:
Laboratoire de Biophysique Moléculaire, Institut de Biologie Structurale J-P Ebel CEA CNRS UJF, Grenoble, France.