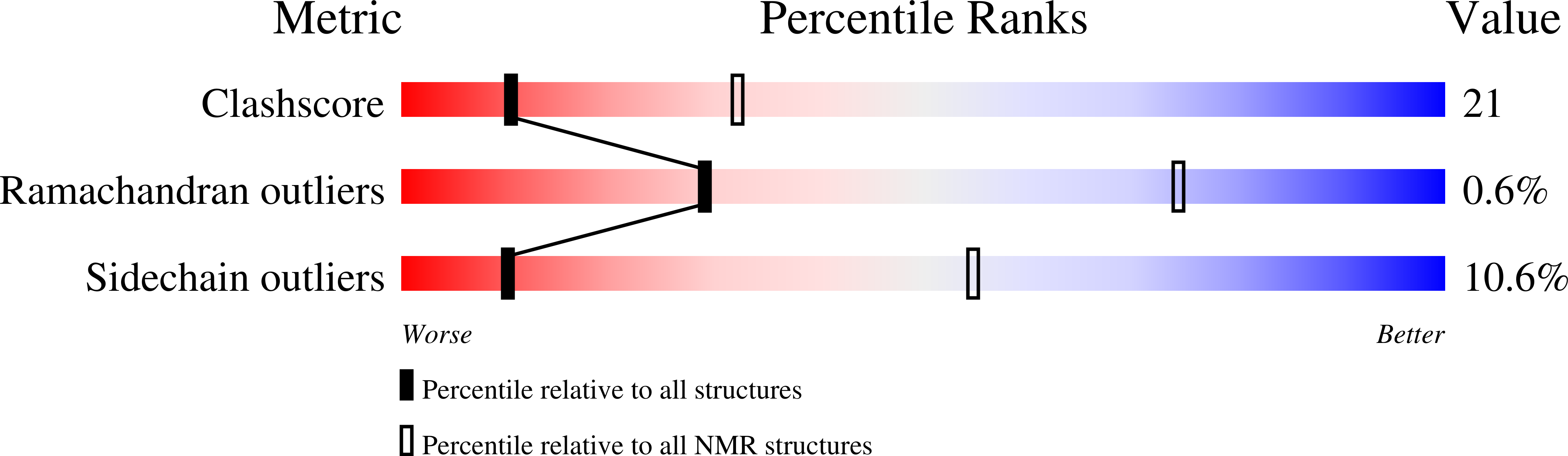A common substrate recognition mode conserved between katanin P60 and VPS4 governs microtubule severing and membrane skeleton reorganization
Iwaya, N., Kuwahara, Y., Fujiwara, Y., Goda, N., Tenno, T., Akiyama, K., Mase, S., Tochio, H., Ikegami, T., Shirakawa, M., Hiroaki, H.(2010) J Biol Chem 285: 16822-16829
- PubMed: 20339000
- DOI: https://doi.org/10.1074/jbc.M110.108365
- Primary Citation of Related Structures:
2RPA - PubMed Abstract:
Katanin p60 (kp60), a microtubule-severing enzyme, plays a key role in cytoskeletal reorganization during various cellular events in an ATP-dependent manner. We show that a single domain isolated from the N terminus of mouse katanin p60 (kp60-NTD) binds to tubulin. The solution structure of kp60-NTD was determined by NMR. Although their sequence similarities were as low as 20%, the structure of kp60-NTD revealed a striking similarity to those of the microtubule interacting and trafficking (MIT) domains, which adopt anti-parallel three-stranded helix bundle. In particular, the arrangement of helices 2 and 3 is well conserved between kp60-NTD and the MIT domain from Vps4, which is a homologous protein that promotes disassembly of the endosomal sorting complexes required for transport III membrane skeleton complex. Mutation studies revealed that the positively charged surface formed by helices 2 and 3 binds tubulin. This binding mode resembles the interaction between the MIT domain of Vps4 and Vps2/CHMP1a, a component of endosomal sorting complexes required for transport III. Our results show that both the molecular architecture and the binding modes are conserved between two AAA-ATPases, kp60 and Vps4. A common mechanism is evolutionarily conserved between two distinct cellular events, one that drives microtubule severing and the other involving membrane skeletal reorganization.
Organizational Affiliation:
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Kyoto-Daigaku Katsura, Nishikyo-ku, Kyoto 615-8530, Japan.