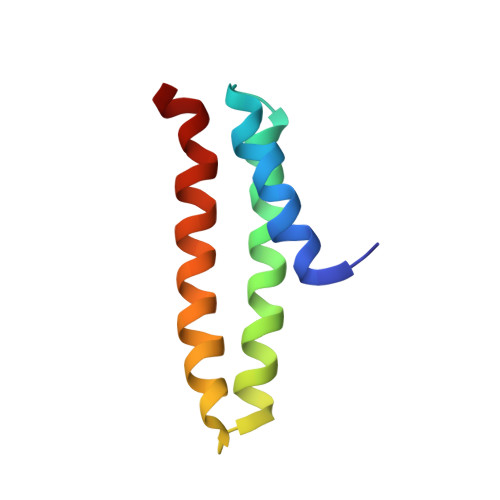
Structural and functional characterization of the microtubule interacting and trafficking domains of two oomycete chitin synthases.
Brown, C., Szpryngiel, S., Kuang, G., Srivastava, V., Ye, W., McKee, L.S., Tu, Y., Maler, L., Bulone, V.(2016) FEBS J 283: 3072-3088
- PubMed: 27363606
- DOI: https://doi.org/10.1111/febs.13794
- Primary Citation of Related Structures:
2MPK - PubMed Abstract:
Chitin synthases (Chs) are responsible for the synthesis of chitin, a key structural cell wall polysaccharide in many organisms. They are essential for growth in certain oomycete species, some of which are pathogenic to diverse higher organisms. Recently, a microtubule interacting and trafficking (MIT) domain, which is not found in any fungal Chs, has been identified in some oomycete Chs proteins. Based on experimental data relating to the binding specificity of other eukaryotic MIT domains, there was speculation that this domain may be involved in the intracellular trafficking of Chs proteins. However, there is currently no evidence for this or any other function for the MIT domain in these enzymes. To attempt to elucidate their function, MIT domains from two Chs enzymes from the oomycete Saprolegnia monoica were cloned, expressed, and characterized. Both were shown to interact strongly with the plasma membrane component, phosphatidic acid, and to have additional putative interactions with proteins thought to be involved in protein transport and localization. Aiding our understanding of these data, the structure of the first MIT domain from a carbohydrate-active enzyme (MIT1) was solved by NMR, and a model structure of a second MIT domain (MIT2) was built by homology modeling. Our results suggest a potential function for these MIT domains in the intracellular transport and/or regulation of Chs enzymes in the oomycetes.
Organizational Affiliation:
Division of Glycoscience, School of Biotechnology, Royal Institute of Technology (KTH), AlbaNova University Centre, Stockholm, Sweden.