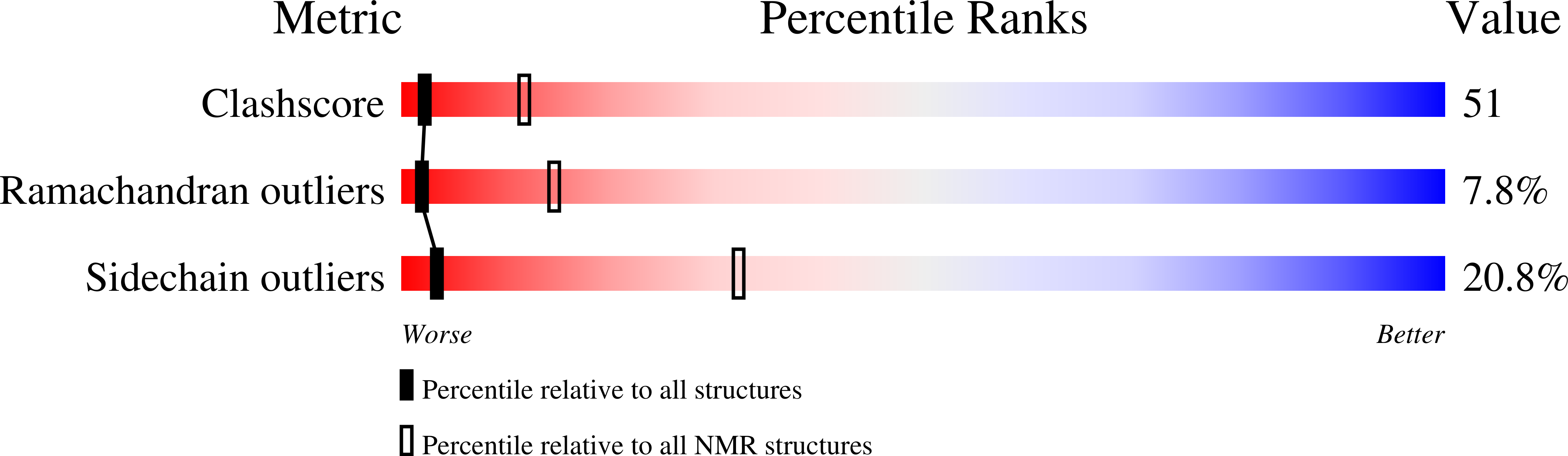Rational design of alpha-helix-stabilized exendin-4 analogues.
Rovo, P., Farkas, V., Straner, P., Szabo, M., Jermendy, A., Hegyi, O., Toth, G.K., Perczel, A.(2014) Biochemistry 53: 3540-3552
- PubMed: 24828921
- DOI: https://doi.org/10.1021/bi500033c
- Primary Citation of Related Structures:
2MJ9 - PubMed Abstract:
Exendin-4 (Ex4) is a potent glucagon-like peptide-1 receptor agonist, a drug regulating the plasma glucose level of patients suffering from type 2 diabetes. The molecule's poor solubility and its readiness to form aggregates increase the likelihood of unwanted side effects. Therefore, we designed Ex4 analogues with improved structural characteristics and better water solubility. Rational design was started from the parent 20-amino acid, well-folded Trp cage (TC) miniprotein and involved the step-by-step N-terminal elongation of the TC head, resulting in the 39-amino acid Ex4 analogue, E19. Helical propensity coupled to tertiary structure compactness was monitored and quantitatively analyzed by electronic circular dichroism and nuclear magnetic resonance (NMR) spectroscopy for the 14 peptides of different lengths. Both (15)N relaxation- and diffusion-ordered NMR measurements were established to investigate the inherent mobility and self-association propensity of Ex4 and E19. Our designed E19 molecule has the same tertiary structure as Ex4 but is more helical than Ex4 under all studied conditions; it is less prone to oligomerization and has preserved biological activity. These conditions make E19 a perfect lead compound for further drug discovery. We believe that this structural study improves our understanding of the relationship between local molecular features and global physicochemical properties such as water solubility and could help in the development of more potent Ex4 analogues with improved pharmacokinetic properties.
Organizational Affiliation:
Laboratory of Structural Chemistry and Biology, Institute of Chemistry, Eötvös Loránd University , Budapest, Hungary.