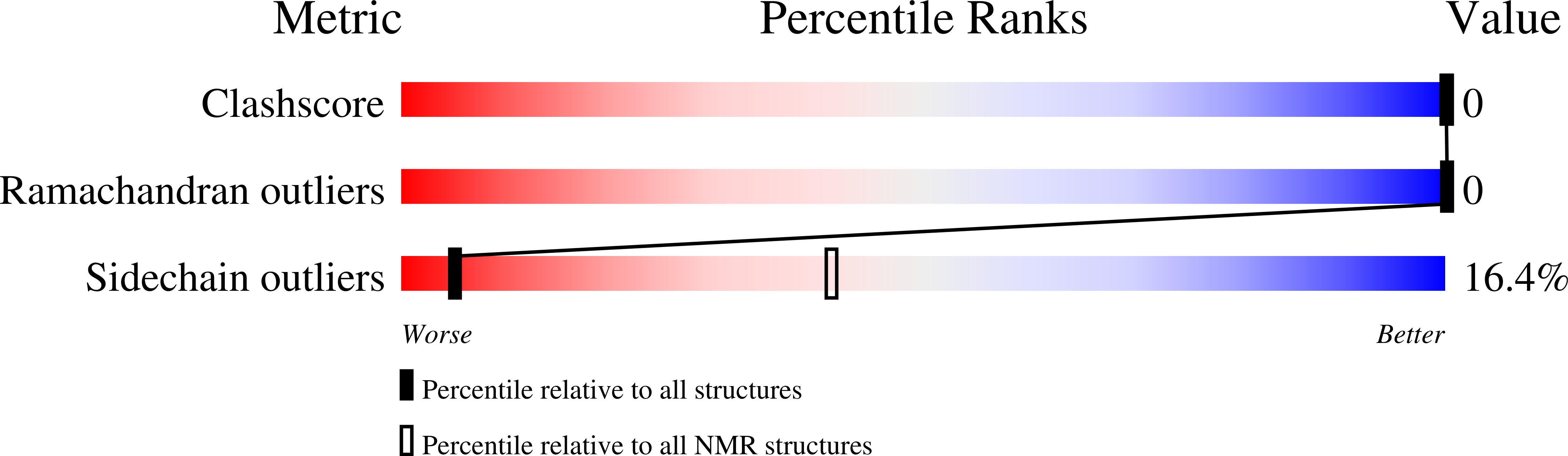Structure and Immunogenicity of a Peptide Vaccine, Including the Complete HIV-1 gp41 2F5 Epitope: IMPLICATIONS FOR ANTIBODY RECOGNITION MECHANISM AND IMMUNOGEN DESIGN.
Serrano, S., Araujo, A., Apellaniz, B., Bryson, S., Carravilla, P., de la Arada, I., Huarte, N., Rujas, E., Pai, E.F., Arrondo, J.L., Domene, C., Jimenez, M.A., Nieva, J.L.(2014) J Biol Chem 289: 6565-6580
- PubMed: 24429284
- DOI: https://doi.org/10.1074/jbc.M113.527747
- Primary Citation of Related Structures:
2M8M, 2M8O - PubMed Abstract:
The membrane-proximal external region (MPER) of gp41 harbors the epitope recognized by the broadly neutralizing anti-HIV 2F5 antibody, a research focus in HIV-1 vaccine development. In this work, we analyze the structure and immunogenic properties of MPERp, a peptide vaccine that includes the following: (i) the complete sequence protected from proteolysis by the 2F5 paratope; (ii) downstream residues postulated to establish weak contacts with the CDR-H3 loop of the antibody, which are believed to be crucial for neutralization; and (iii) an aromatic rich anchor to the membrane interface. MPERp structures solved in dodecylphosphocholine micelles and 25% 1,1,1,3,3,3-hexafluoro-2-propanol (v/v) confirmed folding of the complete 2F5 epitope within continuous kinked helices. Infrared spectroscopy (IR) measurements demonstrated the retention of main helical conformations in immunogenic formulations based on alum, Freund's adjuvant, or two different types of liposomes. Binding to membrane-inserted MPERp, IR, molecular dynamics simulations, and characterization of the immune responses further suggested that packed helical bundles partially inserted into the lipid bilayer, rather than monomeric helices adsorbed to the membrane interface, could encompass effective MPER peptide vaccines. Together, our data constitute a proof-of-concept to support MPER-based peptides in combination with liposomes as stand-alone immunogens and suggest new approaches for structure-aided MPER vaccine development.
Organizational Affiliation:
Institute of Physical Chemistry "Rocasolano," Consejo Superior de Investigaciones Científicas (IQFR-CSIC), Serrano 119, E-28006 Madrid, Spain.