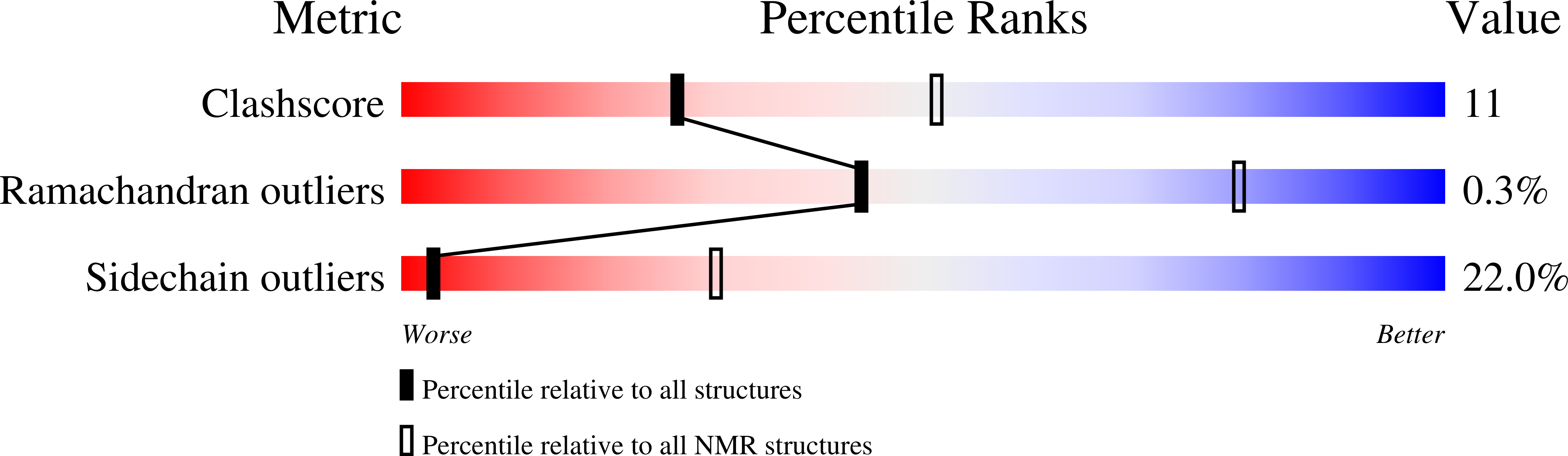The CW domain, a new histone recognition module in chromatin proteins.
Hoppmann, V., Thorstensen, T., Kristiansen, P.E., Veiseth, S.V., Rahman, M.A., Finne, K., Aalen, R.B., Aasland, R.(2011) EMBO J 30: 1939-1952
- PubMed: 21522130
- DOI: https://doi.org/10.1038/emboj.2011.108
- Primary Citation of Related Structures:
2L7P - PubMed Abstract:
Post-translational modifications of the N-terminal histone tails, including lysine methylation, have key roles in regulation of chromatin and gene expression. A number of protein modules have been identified that recognize differentially modified histone tails and provide their proteins with the capacity to sense such modifications. Here, we identify the CW domain of plant and animal chromatin-related proteins as a novel module that recognizes different methylated states of lysine 4 on histone H3 (H3K4me). The solution structure of the CW domain of the Arabidopsis ASH1 HOMOLOG2 (ASHH2) histone methyltransferase provides insight into how different CW domains can distinguish different methylated histone tails. We provide evidence that ASHH2 is acting on H3K4me-marked genes, allowing for ASHH2-dependent H3K36 tri-methylation, which contributes to sustained expression of tissue-specific and developmentally regulated genes. This suggests that ASHH2 is a combined 'reader' and 'writer' of the histone code. We propose that different CW domains, dependent on their specificity for different H3K4 methylations, are important for epigenetic memory or participate in switching between permissive and repressive chromatin states.
Organizational Affiliation:
Department of Molecular Biology, University of Bergen, Bergen, Norway.