Structural biology of human cannabinoid receptor-2 helix 6 in membrane-mimetic environments.
Tiburu, E.K., Tyukhtenko, S., Deshmukh, L., Vinogradova, O., Janero, D.R., Makriyannis, A.(2009) Biochem Biophys Res Commun 384: 243-248
- PubMed: 19397896
- DOI: https://doi.org/10.1016/j.bbrc.2009.04.099
- Primary Citation of Related Structures:
2KI9 - PubMed Abstract:
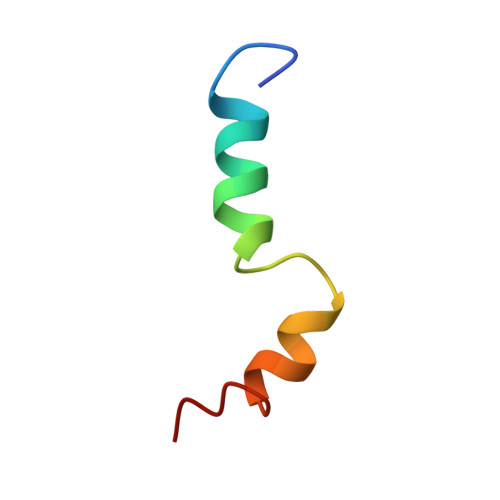
We detail the structure and dynamics of a synthetic peptide corresponding to transmembrane helix 6 (TMH6) of human cannabinoid receptor-2 (hCB2) in biomembrane-mimetic environments. The peptide's NMR structural biology is characterized by two alpha-helical domains bridged by a flexible, nonhelical hinge region containing a highly-conserved CWFP motif with an environmentally sensitive, Pro-based conformational switch. Buried within the peptide's flexible region, W(258) may hydrogen-bond with L(255) to help stabilize the Pro-kinked hCB2 TMH6 structure and position C(257) advantageously for interaction with agonist ligands. These characteristics of hCB2 TMH6 are potential structural features of ligand-induced hCB2 activation in vivo.
Organizational Affiliation:
Center for Drug Discovery and Department of Chemistry and Chemical Biology, Northeastern University, Boston, MA 02115-5000, USA.