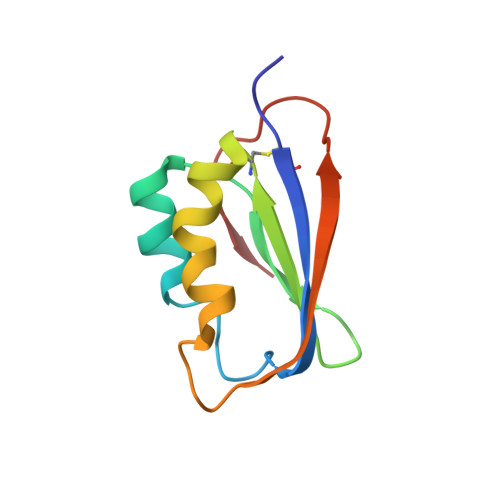
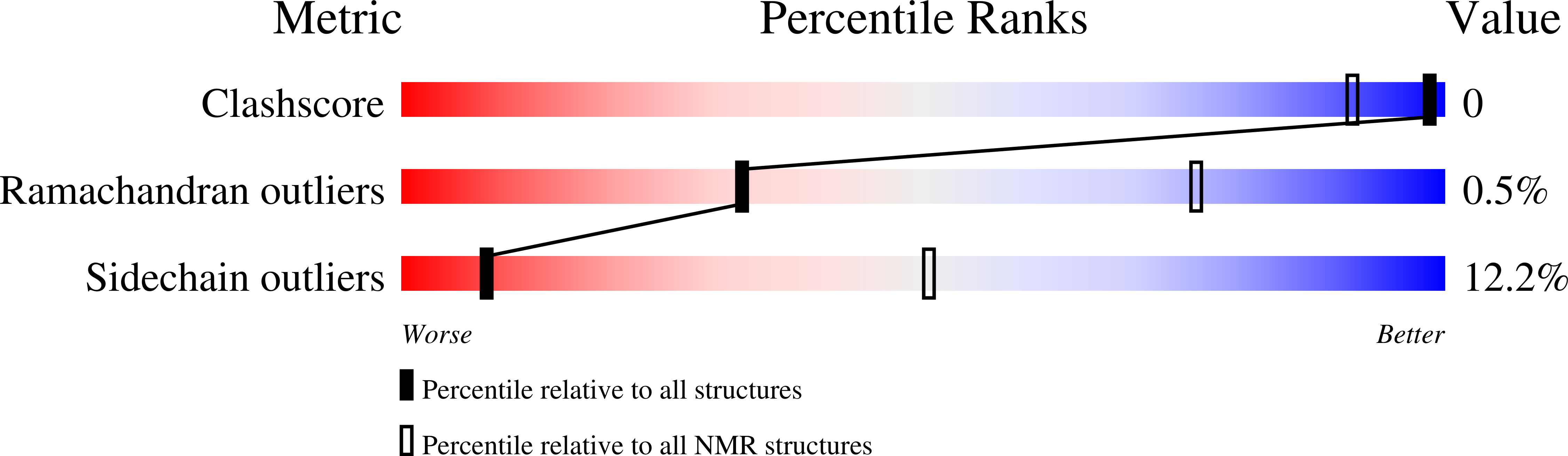NMR solution structure of the acylphosphatase from Escherichia coli.
Pagano, K., Ramazzotti, M., Viglino, P., Esposito, G., Degl'innocenti, D., Taddei, N., Corazza, A.(2006) J Biomol NMR 36: 199-204
- PubMed: 17021943
- DOI: https://doi.org/10.1007/s10858-006-9073-2
- Primary Citation of Related Structures:
2GV1 - PubMed Abstract:
The solution structure of Escherichia coli acylphosphatase (E. coli AcP), a small enzyme catalyzing the hydrolysis of acylphosphates, was determined by (1)H and (15)N NMR and restrained modelling calculation. In analogy with the other members of AcP family, E. coli AcP shows an alpha/beta sandwich domain composed of four antiparallel and one parallel beta-strand, assembled in a five-stranded beta-sheet facing two antiparallel alpha-helices. The pairwise RMSD values calculated for the backbone atoms of E. coli and Sulfolobus solfataricus AcP, Bovine common type AcP and Horse muscle AcP are 2.18, 5.31 and 5.12 A, respectively. No significant differences are present in the active site region and the catalytic residue side chains are consistently positioned in the structures.
Organizational Affiliation:
Department of Biomedical Sciences and Technologies, University of Udine, Udine, Italy.