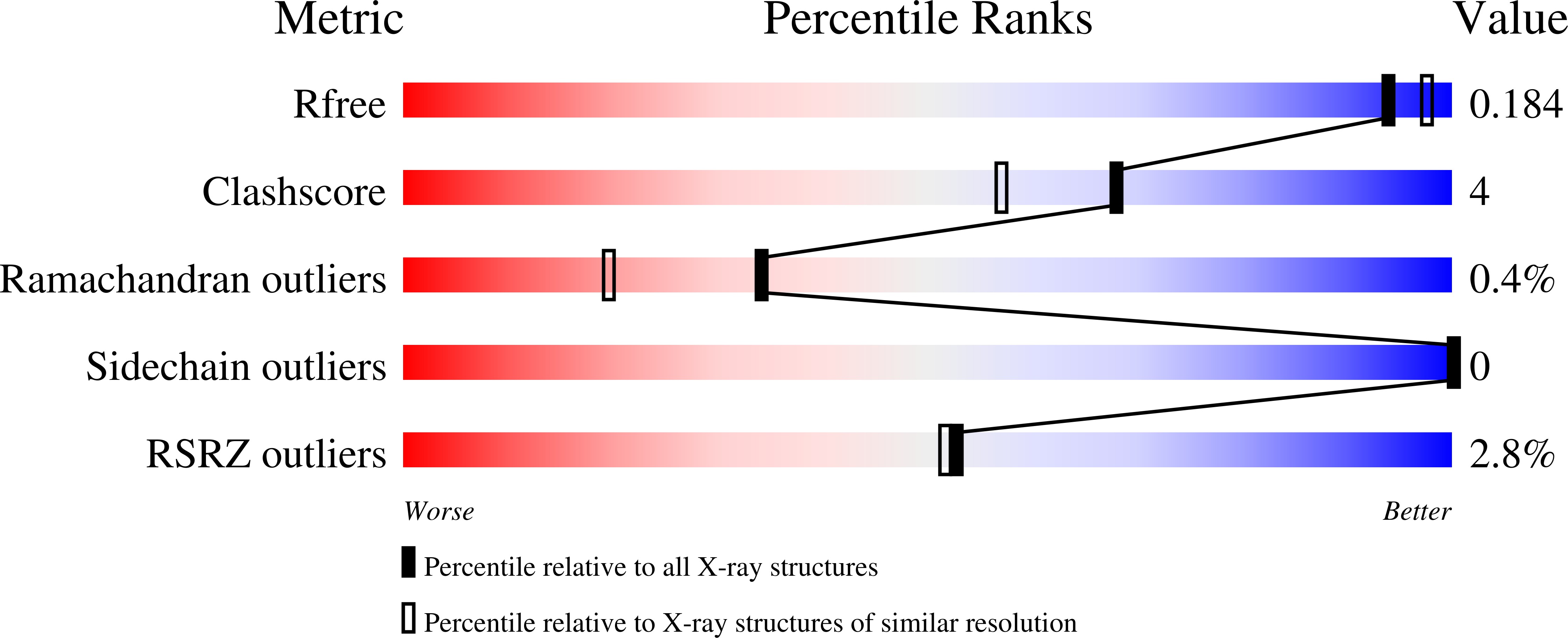
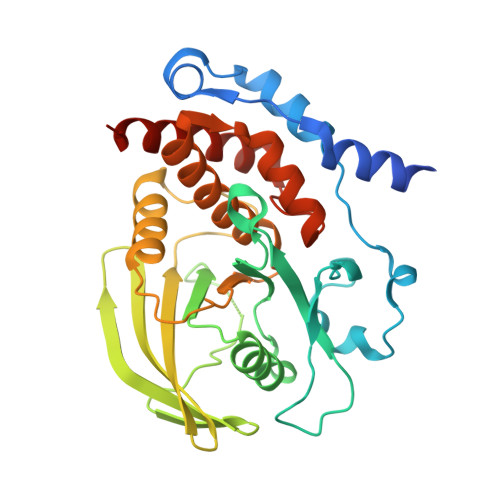
Structure of the hematopoietic tyrosine phosphatase (HePTP) catalytic domain: structure of a KIM phosphatase with phosphate bound at the active site.
Mustelin, T., Tautz, L., Page, R.(2005) J Mol Biol 354: 150-163
- PubMed: 16226275
- DOI: https://doi.org/10.1016/j.jmb.2005.09.049
- Primary Citation of Related Structures:
1ZC0 - PubMed Abstract:
Hematopoietic tyrosine phosphatase (HePTP) is a 38kDa class I non-receptor protein tyrosine phosphatase (PTP) that is strongly expressed in T cells. It is composed of a C-terminal classical PTP domain (residues 44-339) and a short N-terminal extension (residues 1-43) that functions to direct HePTP to its physiological substrates. Moreover, HePTP is a member of a recently identified family of PTPs that has a major role in regulating the activity and translocation of the MAP kinases Erk and p38. HePTP binds Erk and p38 via a short, highly conserved motif in its N terminus, termed the kinase interaction motif (KIM). Association of HePTP with Erk via the KIM results in an unusual, reciprocal interaction between the two proteins. First, Erk phosphorylates HePTP at residues Thr45 and Ser72. Second, HePTP dephosphorylates Erk at PTyr185. In order to gain further insight into the interaction of HePTP with Erk, we determined the structure of the PTP catalytic domain of HePTP, residues 44-339. The HePTP catalytic phosphatase domain displays the classical PTP1B fold and superimposes well with PTP-SL, the first KIM-containing phosphatase solved to high resolution. In contrast to the PTP-SL structure, however, HePTP crystallized with a well-ordered phosphate ion bound at the active site. This resulted in the closure of the catalytically important WPD loop, and thus, HePTP represents the first KIM-containing phosphatase solved in the closed conformation. Finally, using this structure of the HePTP catalytic domain, we show that both the phosphorylation of HePTP at Thr45 and Ser72 by Erk2 and the dephosphorylation of Erk2 at Tyr185 by HePTP require significant conformational changes in both proteins.
Organizational Affiliation:
Program of Inflammation, The Burnham Institute, 10901 N. Torrey Pines Rd., La Jolla, CA 92037, USA.