Activation process of [NiFe] hydrogenase elucidated by high-resolution X-Ray analyses: conversion of the ready to the unready state
Ogata, H., Hirota, S., Nakahara, A., Komori, H., Shibata, N., Kato, T., Kano, K., Higuchi, Y.(2005) Structure 13: 1635-1642
- PubMed: 16271886
- DOI: https://doi.org/10.1016/j.str.2005.07.018
- Primary Citation of Related Structures:
1WUI, 1WUJ, 1WUK, 1WUL - PubMed Abstract:
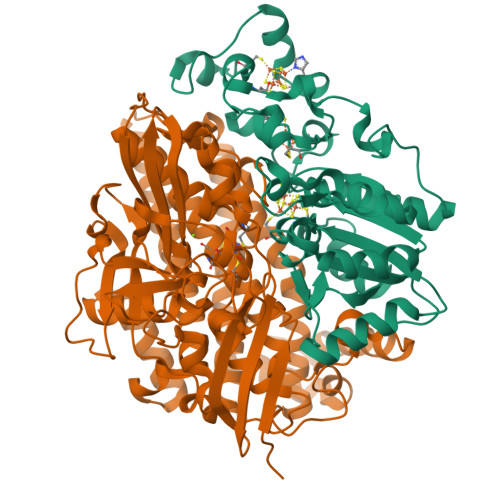
Hydrogenases catalyze oxidoreduction of molecular hydrogen and have potential applications for utilizing dihydrogen as an energy source. [NiFe] hydrogenase has two different oxidized states, Ni-A (unready, exhibits a lag phase in reductive activation) and Ni-B (ready). We have succeeded in converting Ni-B to Ni-A with the use of Na2S and O2 and determining the high-resolution crystal structures of both states. Ni-B possesses a monatomic nonprotein bridging ligand at the Ni-Fe active site, whereas Ni-A has a diatomic species. The terminal atom of the bridging species of Ni-A occupies a similar position as C of the exogenous CO in the CO complex (inhibited state). The common features of the enzyme structures at the unready (Ni-A) and inhibited (CO complex) states are proposed. These findings provide useful information on the design of new systems of biomimetic dihydrogen production and fuel cell devices.
Organizational Affiliation:
Department of Life Science, Graduate School of Life Science, University of Hyogo and Himeji Institute of Technology, 3-2-1 Koto, Ako-gun, Hyogo 678-1297, Japan.