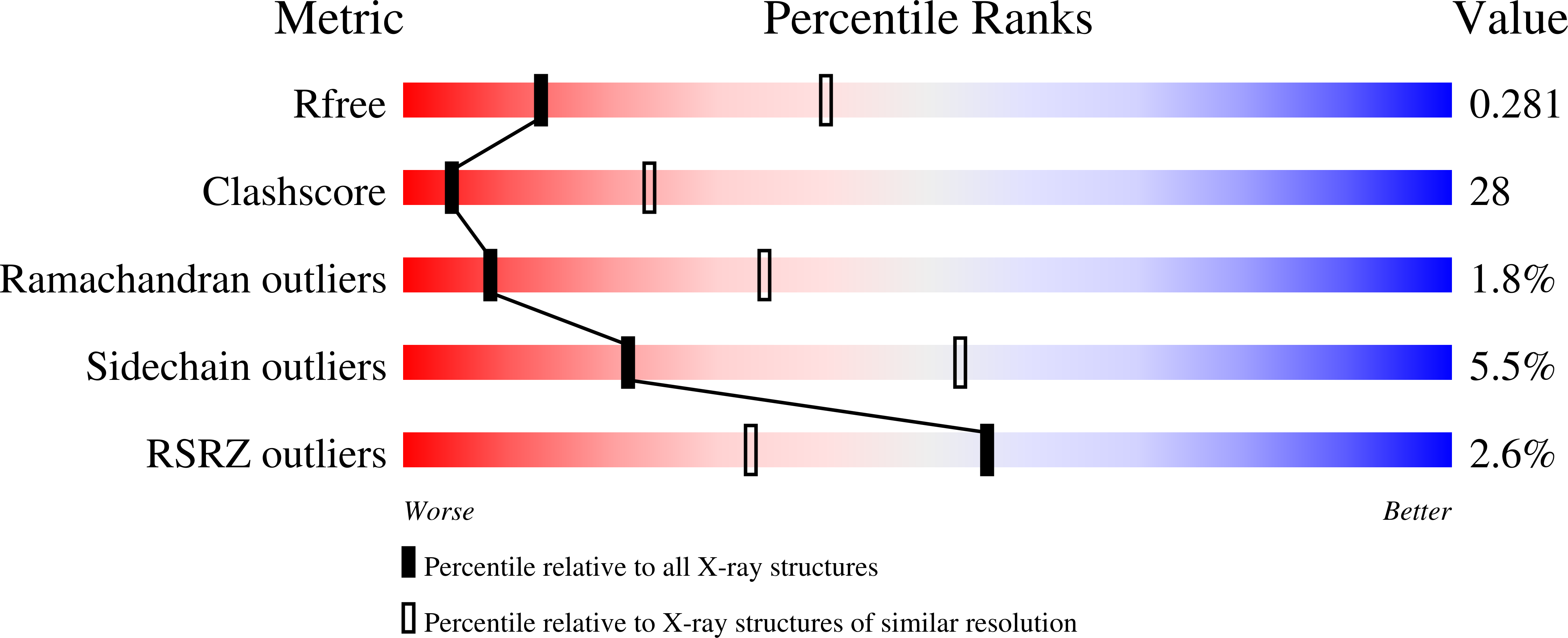
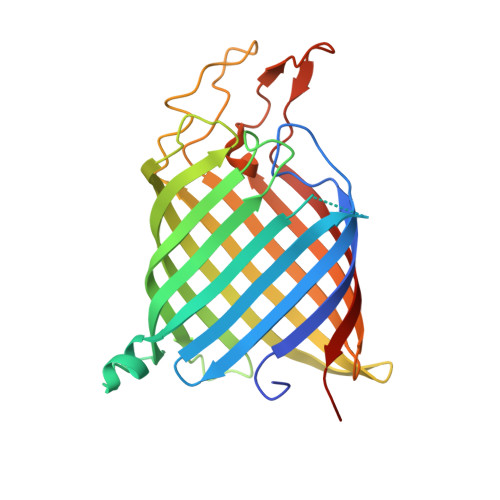
Crystal structure of the bacterial nucleoside transporter Tsx.
Ye, J., Van Den Berg, B.(2004) EMBO J 23: 3187-3195
- PubMed: 15272310
- DOI: https://doi.org/10.1038/sj.emboj.7600330
- Primary Citation of Related Structures:
1TLW, 1TLY, 1TLZ - PubMed Abstract:
Tsx is a nucleoside-specific outer membrane (OM) transporter of Gram-negative bacteria. We present crystal structures of Escherichia coli Tsx in the absence and presence of nucleosides. These structures provide a mechanism for nucleoside transport across the bacterial OM. Tsx forms a monomeric, 12-stranded beta-barrel with a long and narrow channel spanning the outer membrane. The channel, which is shaped like a keyhole, contains several distinct nucleoside-binding sites, two of which are well defined. The base moiety of the nucleoside is located in the narrow part of the keyhole, while the sugar occupies the wider opening. Pairs of aromatic residues and flanking ionizable residues are involved in nucleoside binding. Nucleoside transport presumably occurs by diffusion from one binding site to the next.
Organizational Affiliation:
Department of Cell Biology, Howard Hughes Medical Institute and Harvard Medical School, Boston, MA 02115, USA.