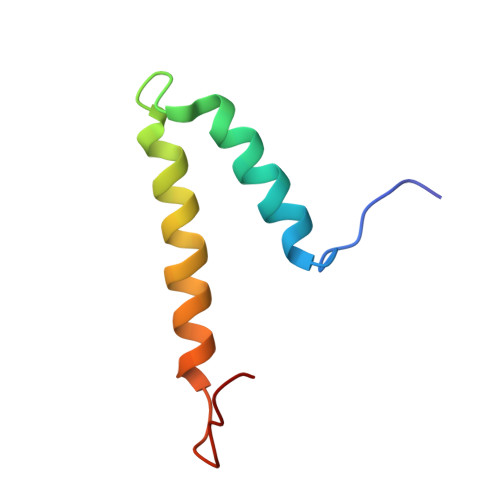
The structure of docking domains in modular polyketide synthases.
Broadhurst, R.W., Nietlispach, D., Wheatcroft, M.P., Leadlay, P.F., Weissman, K.J.(2003) Chem Biol 10: 723-731
- PubMed: 12954331
- DOI: https://doi.org/10.1016/s1074-5521(03)00156-x
- Primary Citation of Related Structures:
1PZQ, 1PZR - PubMed Abstract:
Polyketides from actinomycete bacteria provide the basis for many valuable medicines, so engineering genes for their biosynthesis to produce variant molecules holds promise for drug discovery. The modular polyketide synthases are particularly amenable to this approach, because each cycle of chain extension is catalyzed by a different module of enzymes, and the modules are arranged within giant multienzyme subunits in the order in which they act. Protein-protein interactions between terminal docking domains of successive multienzymes promote their correct positioning within the assembly line, but because the overall complex is not stable in vitro, the key interactions have not been identified. We present here the NMR solution structure of a 120 residue polypeptide representing a typical pair of such domains, fused at their respective C and N termini: it adopts a stable dimeric structure which reveals the detailed role of these (predominantly helical) domains in docking and dimerization by modular polyketide synthases.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, 80 Tennis Court Road, CB2 1GA, Cambridge, United Kingdom.