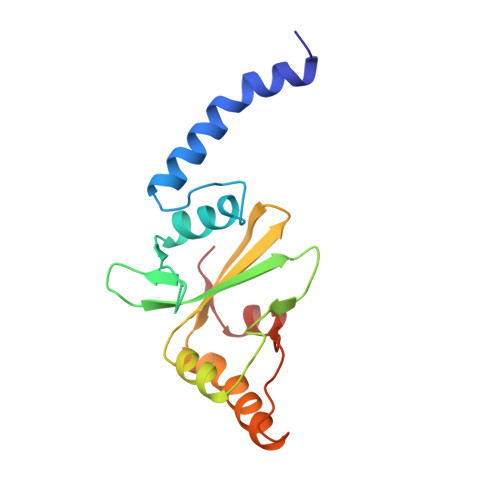
Crystal structure of PvuII endonuclease reveals extensive structural homologies to EcoRV.
Athanasiadis, A., Vlassi, M., Kotsifaki, D., Tucker, P.A., Wilson, K.S., Kokkinidis, M.(1994) Nat Struct Biol 1: 469-475
- PubMed: 7664066
- DOI: https://doi.org/10.1038/nsb0794-469
- Primary Citation of Related Structures:
1PVU - PubMed Abstract:
The crystal structure of the dimeric PvuII restriction endonuclease (R.PvuII) has been determined at a resolution of 2.4A. The protein has a mixed alpha/beta architecture and consists of two subdomains. Despite a lack of sequence homology, extensive structural similarities exist between one R.PvuII subdomain and the DNA-binding subdomain of EcoRV endonuclease (R.EcoRV); the dimerization subdomains are unrelated. Within the similar domains, flexible segments of R.PvuII are topologically equivalent to the DNA-binding turns of R.EcoRV; potential catalytic residues can be deduced from the structural similarities to R.EcoRV. Conformational flexibility is important for the interaction with DNA. A possible classification of endonuclease structures on the basis of the positions of the scissile phosphates is discussed.
Organizational Affiliation:
Department of Biology, University of Crete, Greece.