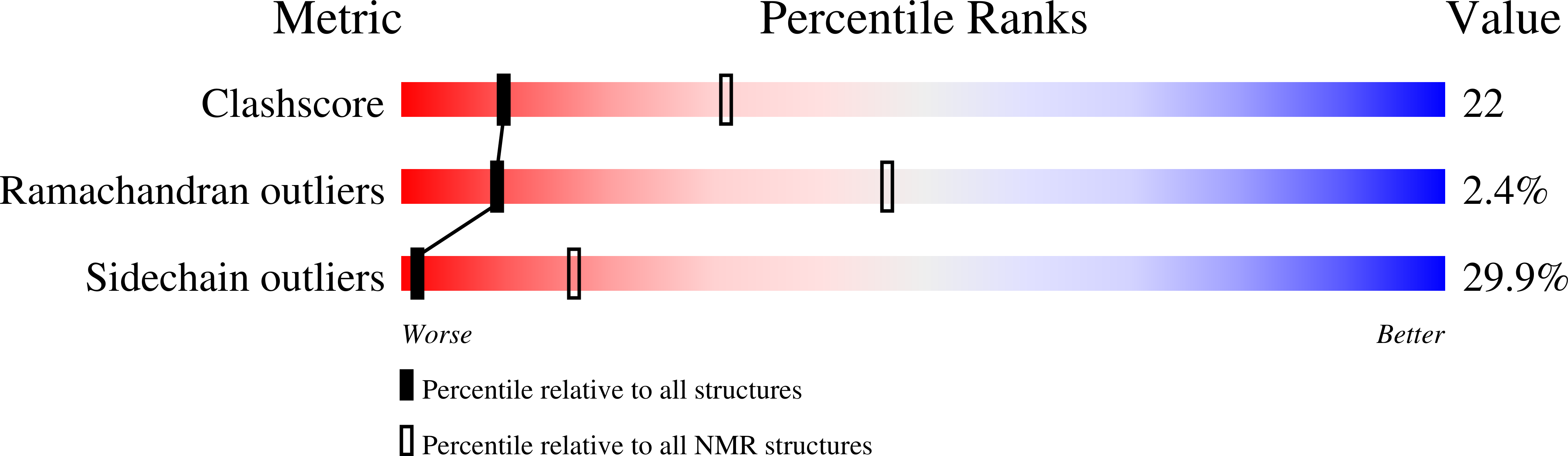Solution structure of the DNA-binding domain of NtrC with three alanine substitutions.
Pelton, J.G., Kustu, S., Wemmer, D.E.(1999) J Mol Biol 292: 1095-1110
- PubMed: 10512705
- DOI: https://doi.org/10.1006/jmbi.1999.3140
- Primary Citation of Related Structures:
1NTC - PubMed Abstract:
The structure of the 20 kDa C-terminal DNA-binding domain of NtrC from Salmonella typhimurium (residues Asp380-Glu469) with alanine replacing Arg456, Asn457, and Arg461, was determined by NMR spectroscopy. NtrC is a homodimeric enhancer-binding protein that activates the transcription of genes whose products are required for nitrogen metabolism. The 91-residue C-terminal domain contains the determinants necessary for dimerization and DNA-binding of the full length protein. The mutant protein does not bind to DNA but retains many characteristics of the wild-type protein, and the mutant domain expresses at high yield (20 mg/l) in minimal medium. Three-dimensional (1)H/(13)C/(15)N triple-resonance, (1)H-(13)C-(13)C-(1)H correlation and (15)N-separated nuclear Overhauser effect (NOE) spectroscopy experiments were used to make backbone and side-chain (1)H,(15)N, and (13)C assignments. The structures were calculated using a total of 1580 intra and inter-monomer distance and hydrogen bond restraints (88 hydrogen bonds; 44 hydrogen bond restraints), and 88 phi dihedral restraints for residues Asp400 through Glu469 in both monomers. A total of 54 ambiguous restraints (intra or inter-monomer) involving residues close to the 2-fold symmetry axis were also included. Each monomer consists of four helical segments. Helices A (Trp402-Leu414) and B (Leu421-His440) join with those of another monomer to form an antiparallel four-helix bundle. Helices C (Gln446-Leu451) and D (Ala456-Met468) of each monomer adopt a classic helix-turn-helix DNA-binding fold at either end of the protein. The backbone rms deviation for the 28 best of 40 starting structures is 0.6 (+/-0.2) A. Structural differences between the C-terminal domain of NtrC and the homologous Factor for Inversion Stimulation are discussed.
Organizational Affiliation:
Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94710, USA.