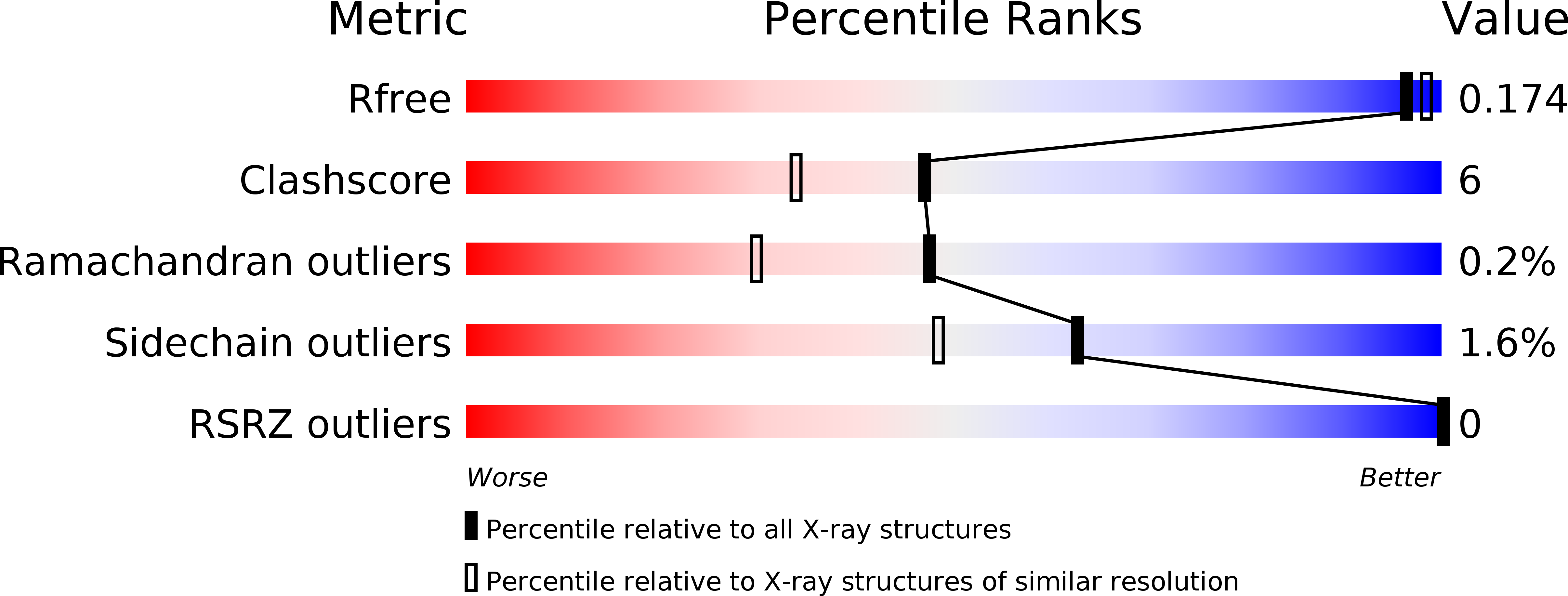
A new UAG-encoded residue in the structure of a methanogen methyltransferase.
Hao, B., Gong, W., Ferguson, T.K., James, C.M., Krzycki, J.A., Chan, M.K.(2002) Science 296: 1462-1466
- PubMed: 12029132
- DOI: https://doi.org/10.1126/science.1069556
- Primary Citation of Related Structures:
1L2Q, 1NTH - PubMed Abstract:
Genes encoding methanogenic methylamine methyltransferases all contain an in-frame amber (UAG) codon that is read through during translation. We have identified the UAG-encoded residue in a 1.55 angstrom resolution structure of the Methanosarcina barkeri monomethylamine methyltransferase (MtmB). This structure reveals a homohexamer comprised of individual subunits with a TIM barrel fold. The electron density for the UAG-encoded residue is distinct from any of the 21 natural amino acids. Instead it appears consistent with a lysine in amide-linkage to (4R,5R)-4-substituted-pyrroline-5-carboxylate. We suggest that this amino acid be named l-pyrrolysine.
Organizational Affiliation:
Department of Biochemistry, The Ohio State University, 484 West 12th Avenue, Columbus, OH 43210, USA.