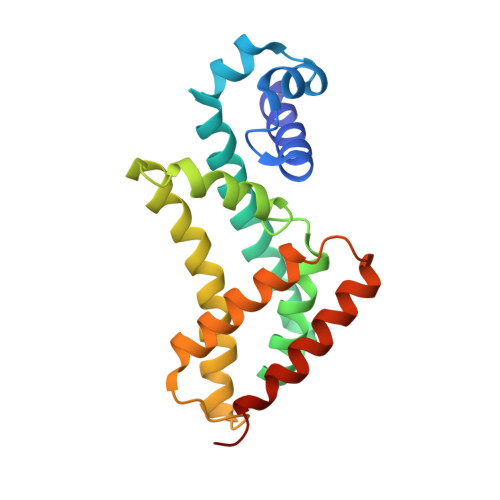
Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR.
Schumacher, M.A., Miller, M.C., Grkovic, S., Brown, M.H., Skurray, R.A., Brennan, R.G.(2002) EMBO J 21: 1210-1218
- PubMed: 11867549
- DOI: https://doi.org/10.1093/emboj/21.5.1210
- Primary Citation of Related Structures:
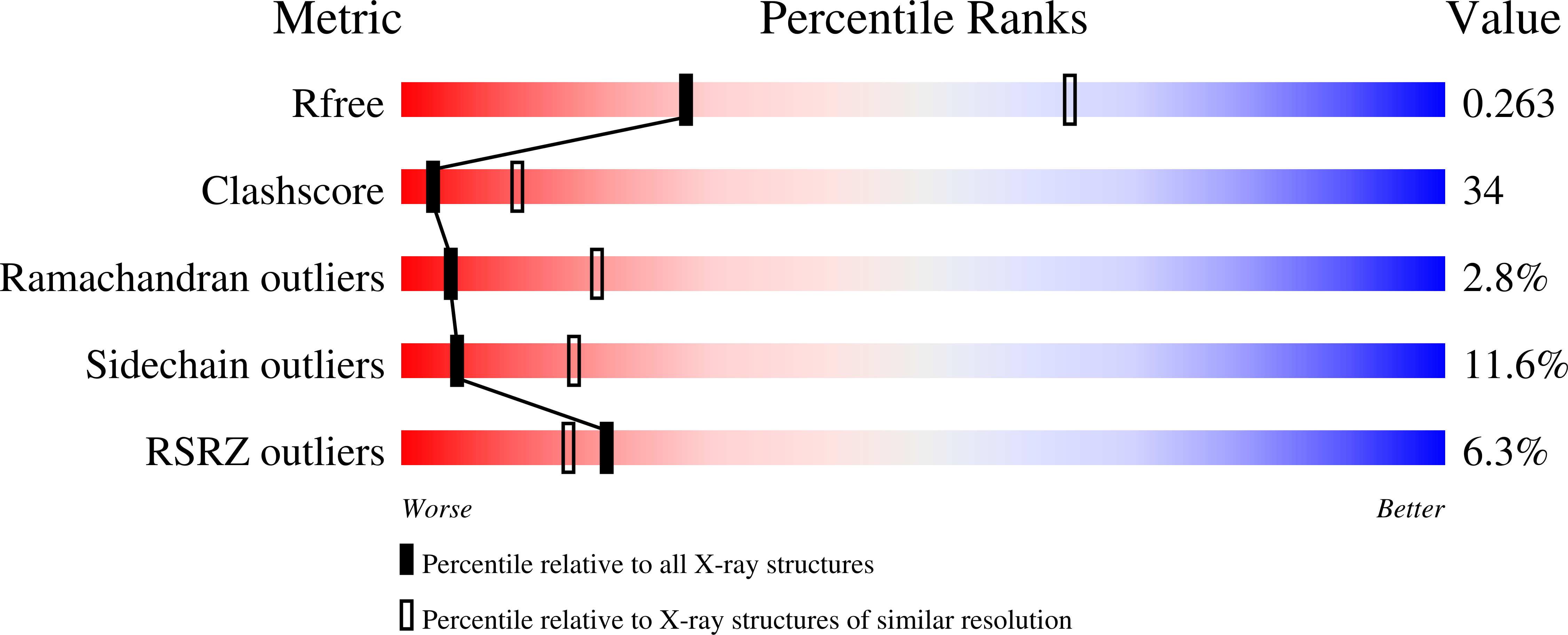
1JT0 - PubMed Abstract:
The Staphylococcus aureus multidrug-binding protein QacR represses transcription of the qacA multidrug transporter gene and is induced by multiple structurally dissimilar drugs. QacR is a member of the TetR/CamR family of transcriptional regulators, which share highly homologous N-terminal DNA-binding domains connected to seemingly non-homologous ligand-binding domains. Unlike other TetR members, which bind approximately 15 bp operators, QacR recognizes an unusually long 28 bp operator, IR1, which it appears to bind cooperatively. To elucidate the DNA-binding mechanism of QacR, we determined the 2.90 A resolution crystal structure of a QacR-IR1 complex. Strikingly, our data reveal that the DNA recognition mode of QacR is distinct from TetR and involves the binding of a pair of QacR dimers. In this unique binding mode, recognition at each IR1 half-site is mediated by a complement of DNA contacts made by two helix-turn-helix motifs. The inferred cooperativity does not arise from cross-dimer protein-protein contacts, but from the global undertwisting and major groove widening elicited by the binding of two QacR dimers.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Oregon Health & Science University, Portland, OR 97201-3098, USA.