Crystal structure of oxygenated Scapharca dimeric hemoglobin at 1.7-A resolution.
Condon, P.J., Royer Jr., W.E.(1994) J Biol Chem 269: 25259-25267
- PubMed: 7929217
- DOI: https://doi.org/10.2210/pdb1hbi/pdb
- Primary Citation of Related Structures:
1HBI - PubMed Abstract:
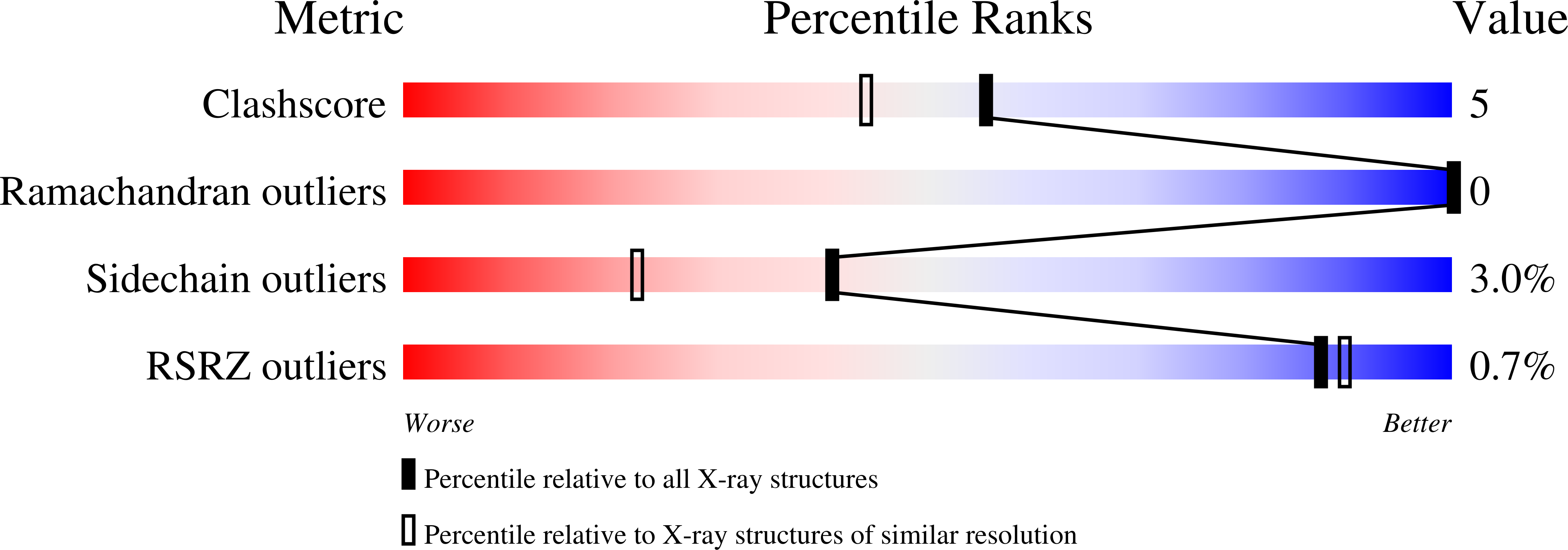
The crystal structure of the cooperative dimeric hemoglobin from the blood clam Scapharca inaequivalvis has been determined in the oxygenated state and refined to an R-factor of 0.157 at 1.7-A resolution. The structure is very similar to the carbon monoxide-liganded form with subtle differences in ligand binding geometry. Oxygen binds to the heme iron in a bent conformation with Fe-O-O angles of 135 degrees and 150 degrees for the two subunits. These observed angles are lower than the equivalent angles in the carbon monoxide-liganded form and intermediate between the angles observed in structures of oxygenated sperm whale myoglobin and oxygenated human hemoglobin. This third high resolution structure of Scapharca dimeric hemoglobin permits a detailed analysis of the water structure in the highly hydrated interface between subunits.
Organizational Affiliation:
Program in Molecular Medicine, University of Massachusetts Medical Center, Worcester 01605.