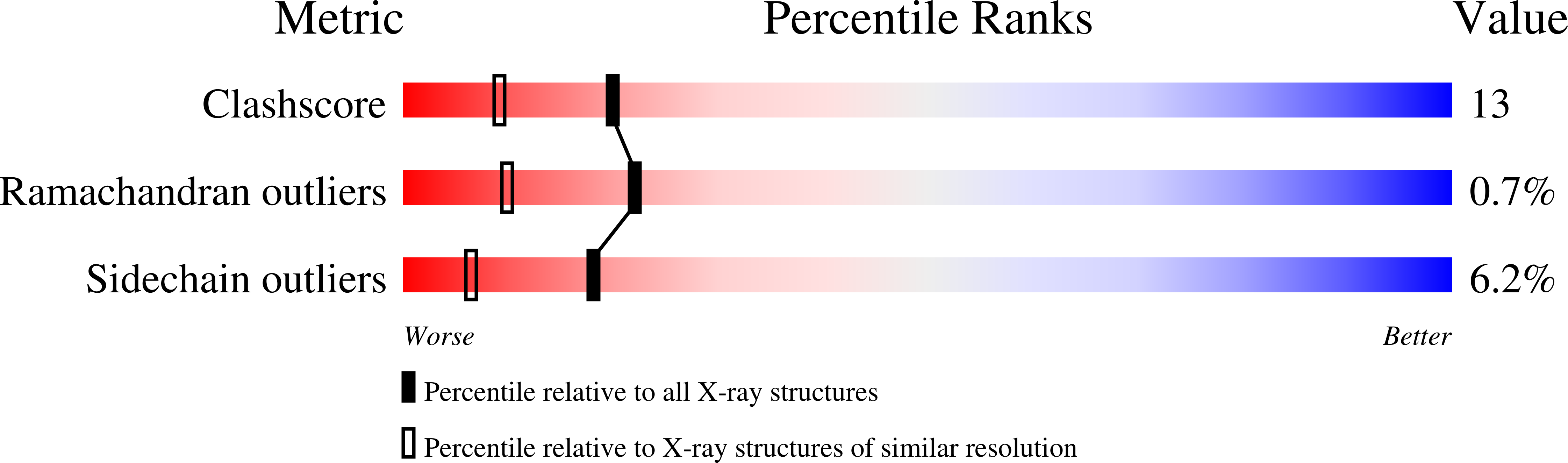Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion.
Ackerman, S.J., Liu, L., Kwatia, M.A., Savage, M.P., Leonidas, D.D., Swaminathan, G.J., Acharya, K.R.(2002) J Biol Chem 277: 14859-14868
- PubMed: 11834744
- DOI: https://doi.org/10.1074/jbc.M200221200
- Primary Citation of Related Structures:
1G86, 1HDK - PubMed Abstract:
Charcot-Leyden crystal (CLC) protein, initially reported to possess weak lysophospholipase activity, is still considered to be the eosinophil's lysophospholipase, but it shows no sequence similarities to any known lysophospholipases. In contrast, CLC protein has moderate sequence similarity, conserved genomic organization, and near structural identity to members of the galectin superfamily, and it has been designated galectin-10. To definitively determine whether or not CLC protein is a lysophospholipase, we reassessed its enzymatic activity in peripheral blood eosinophils and an eosinophil myelocyte cell line (AML14.3D10). Antibody affinity chromatography was used to fully deplete CLC protein from eosinophil lysates. The CLC-depleted lysates retained their full lysophospholipase activity, and this activity could be blocked by sulfhydryl group-reactive inhibitors, N-ethylmaleimide and p-chloromercuribenzenesulfonate, previously reported to inhibit the eosinophil enzyme. In contrast, the affinity-purified CLC protein lacked significant lysophospholipase activity. X-ray crystallographic structures of CLC protein in complex with the inhibitors showed that p-chloromercuribenzenesulfonate bound CLC protein via disulfide bonds with Cys(29) and with Cys(57) near the carbohydrate recognition domain (CRD), whereas N-ethylmaleimide bound to the galectin-10 CRD via ring stacking interactions with Trp(72), in a manner highly analogous to mannose binding to this CRD. Antibodies to rat pancreatic lysophospholipase identified a protein in eosinophil and AML14.3D10 cell lysates, comparable in size with human pancreatic lysophospholipase, which co-purifies in small quantities with CLC protein. Ligand blotting of human and murine eosinophil lysates with CLC protein as probe showed that it binds proteins also recognized by antibodies to pancreatic lysophospholipase. Our results definitively show that CLC protein is not one of the eosinophil's lysophospholipases but that it does interact with eosinophil lysophospholipases and known inhibitors of this lipolytic activity.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Illinois at Chicago, Chicago, Illinois 60612, USA. sackerma@uic.edu