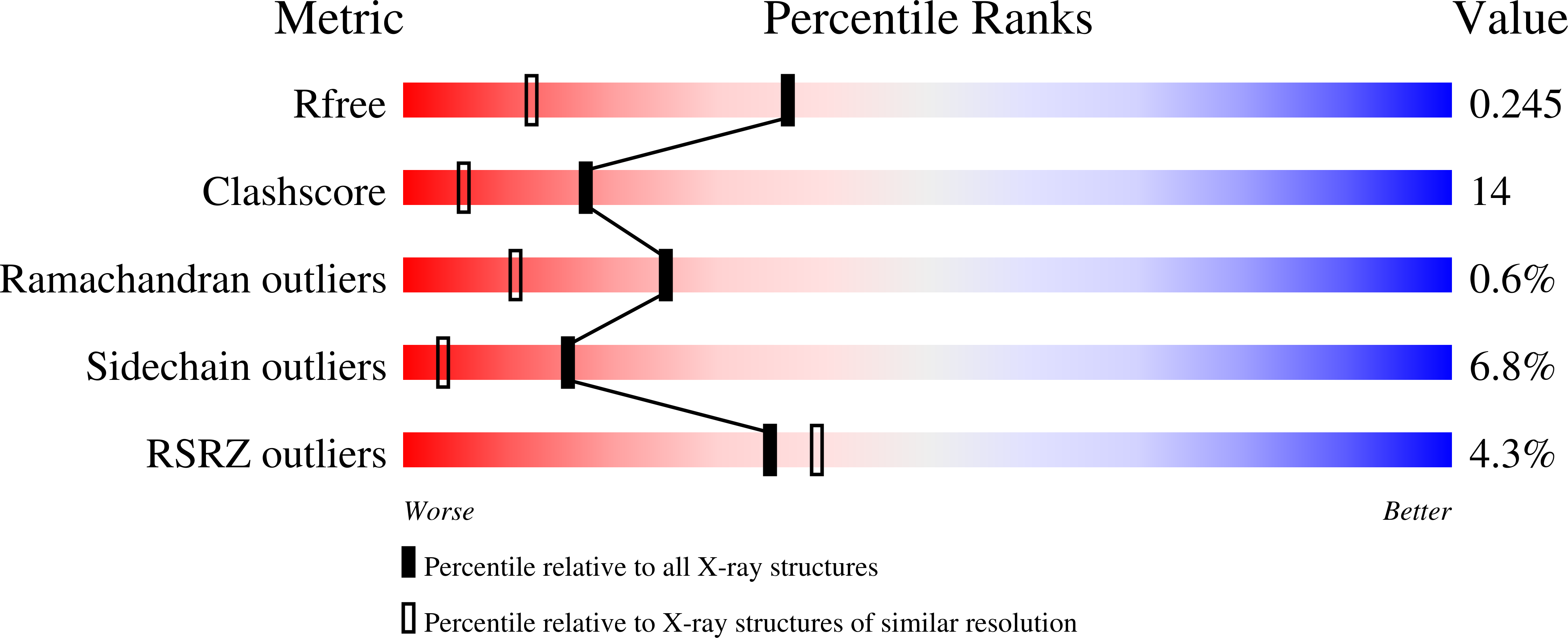
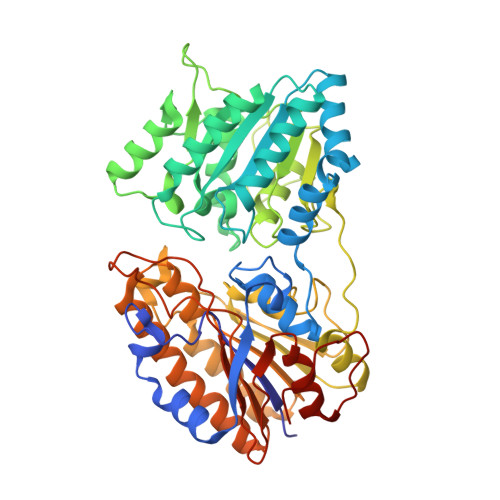
Mechanism of catalysis of the cofactor-independent phosphoglycerate mutase from Bacillus stearothermophilus. Crystal structure of the complex with 2-phosphoglycerate.
Jedrzejas, M.J., Chander, M., Setlow, P., Krishnasamy, G.(2000) J Biol Chem 275: 23146-23153
- PubMed: 10764795
- DOI: https://doi.org/10.1074/jbc.M002544200
- Primary Citation of Related Structures:
1EQJ - PubMed Abstract:
The structure of the complex between the 2, 3-diphosphoglycerate-independent phosphoglycerate mutase (iPGM) from Bacillus stearothermophilus and its 3-phosphoglycerate substrate has recently been solved, and analysis of this structure allowed formulation of a mechanism for iPGM catalysis. In order to obtain further evidence for this mechanism, we have solved the structure of this iPGM complexed with 2-phosphoglycerate and two Mn(2+) ions at 1. 7-A resolution. The structure consists of two different domains connected by two loops and interacting through a network of hydrogen bonds. This structure is consistent with the proposed mechanism for iPGM catalysis, with the two main steps in catalysis being a phosphatase reaction removing the phosphate from 2- or 3-phosphoglycerate, generating an enzyme-bound phosphoserine intermediate, followed by a phosphotransferase reaction as the phosphate is transferred from the enzyme back to the glycerate moiety. The structure also allowed the assignment of the function of the two domains of the enzyme, one of which participates in the phosphatase reaction and formation of the phosphoserine enzyme intermediate, with the other involved in the phosphotransferase reaction regenerating phosphoglycerate. Significant structural similarity has also been found between the active site of the iPGM domain catalyzing the phosphatase reaction and Escherichia coli alkaline phosphatase.
Organizational Affiliation:
Department of Microbiology, University of Alabama at Birmingham, Birmingham, Alabama 35294, USA. jedrzejas@uab.edu