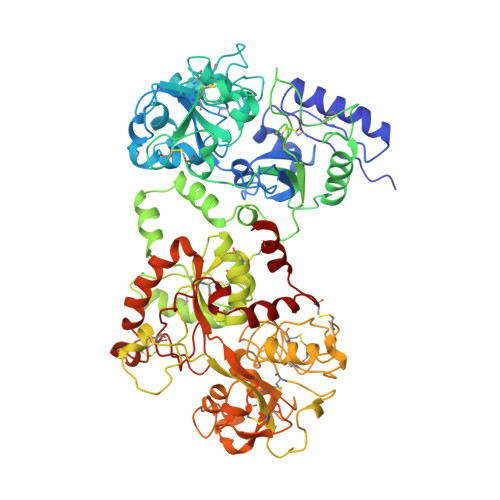
Structure of mare apolactoferrin: the N and C lobes are in the closed form.
Sharma, A.K., Rajashankar, K.R., Yadav, M.P., Singh, T.P.(1999) Acta Crystallogr D Biol Crystallogr 55: 1152-1157
- PubMed: 10329777
- DOI: https://doi.org/10.1107/s0907444999003807
- Primary Citation of Related Structures:
1B7U - PubMed Abstract:
The structure of mare apolactoferrin (MALT) has been determined at 3. 8 A resolution by the molecular-replacement method, using the structure of mare diferric lactoferrin (MDLT) as the search model. The MDLT structure contains two iron-binding sites: one in the N-terminal lobe, lying between domains N1 and N2, and one in the C-terminal lobe between domains C1 and C2. Both lobes have a closed structure. MALT was crystallized using the microdialysis method with 10%(v/v) ethanol in 0.01 M Tris-HCl. The structure has been refined to a final R factor of 0.20 for all data to 3.8 A resolution. Comparison of the structure of MALT with that of MDLT showed that the domain arrangements in these structures are identical. However, the structure of MALT is very different to the structures of human apolactoferrin (HALT) and duck apo-ovotransferrin (DAOT), in which the domain associations differ greatly. In HALT, the N lobe adopts an open conformation while the C lobe is in the closed form. On the other hand, in DAOT both the N and the C lobes adopt the open form. These results indicate the domain arrangements in these proteins to be an important structural feature related to their specific biological functions. Based on the structures of MALT, HALT and DAOT, it can be stated that the native apoproteins of the transferrin family adopt three forms: (i) with both the N and the C lobes in closed forms, as observed in MALT, (ii) with the N lobe open and the C lobe closed, as observed in HALT, and (iii) with both the N and the C lobes open, as found in DAOT. All these proteins attain a convergent form when iron is bound to them, suggesting an efficient and unique form of iron binding. The interface between the N and C lobes, which is formed by N1-C1 contact in the core of the molecule, does not change significantly.
Organizational Affiliation:
Department of Biophysics, All India Institute of Medical Sciences, New Delhi 110 029, India.