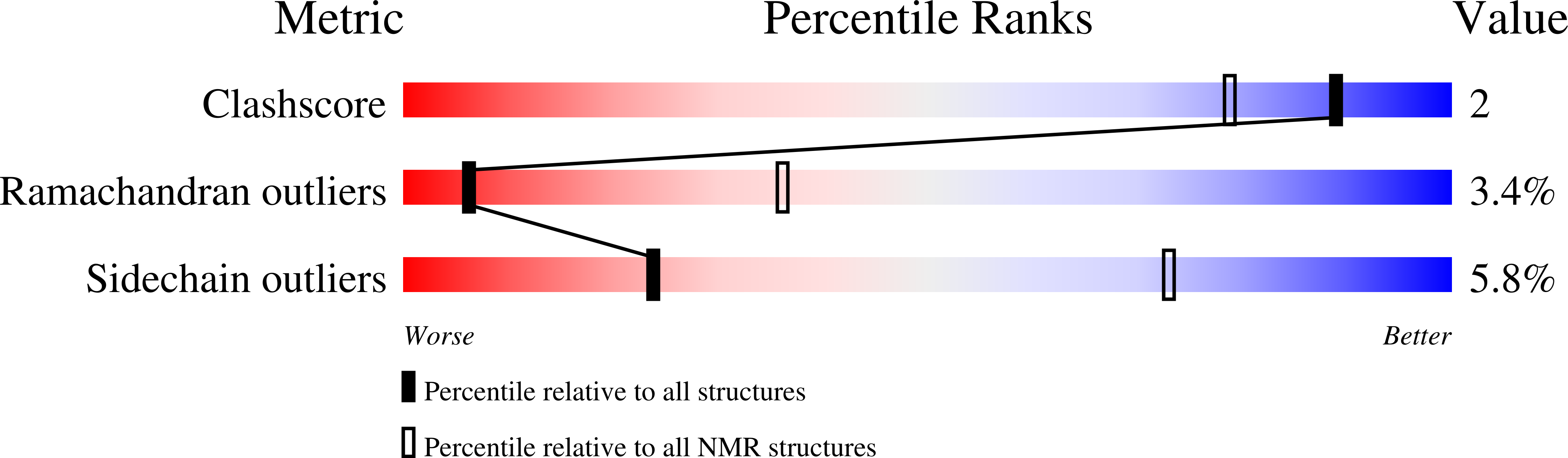
Isolation and structural characterization of epilancin 15X, a novel lantibiotic from a clinical strain of Staphylococcus epidermidis.
Ekkelenkamp, M.B., Hanssen, M., Danny Hsu, S.T., de Jong, A., Milatovic, D., Verhoef, J., van Nuland, N.A.(2005) FEBS Lett 579: 1917-1922
- PubMed: 15792796
- DOI: https://doi.org/10.1016/j.febslet.2005.01.083
- Primary Citation of Related Structures:
1W9N - PubMed Abstract:
The potential application of lantibiotics as food-preserving agents and more recently as antibiotics has strongly increased the interest in these antibacterial peptides. Here, we report the elucidation of the primary and three-dimensional structures of the novel lantibiotic epilancin 15X from Staphylococcus epidermidis using high-resolution nuclear magnetic resonance spectroscopy and tandem mass spectrometry. The molecule contains ten post-translationally modified amino acids, three lanthionine ring structures and a hydroxy-propionyl N-terminal moiety. The primary and tertiary structure and the distribution of positive charges are closely similar to the previously identified lantibiotic epilancin K7, most likely indicative of a common mode of action.
Organizational Affiliation:
University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. M.Ekkelenkamp@lab.azu.nl