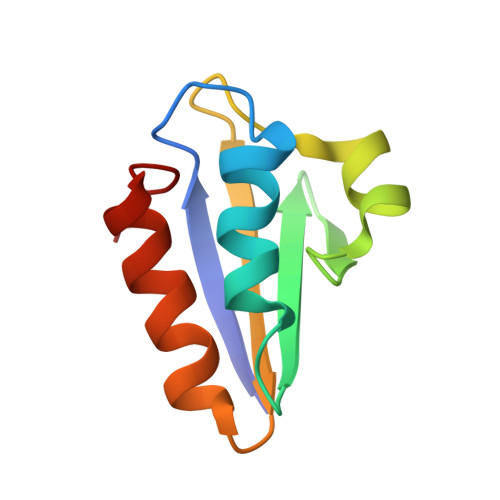
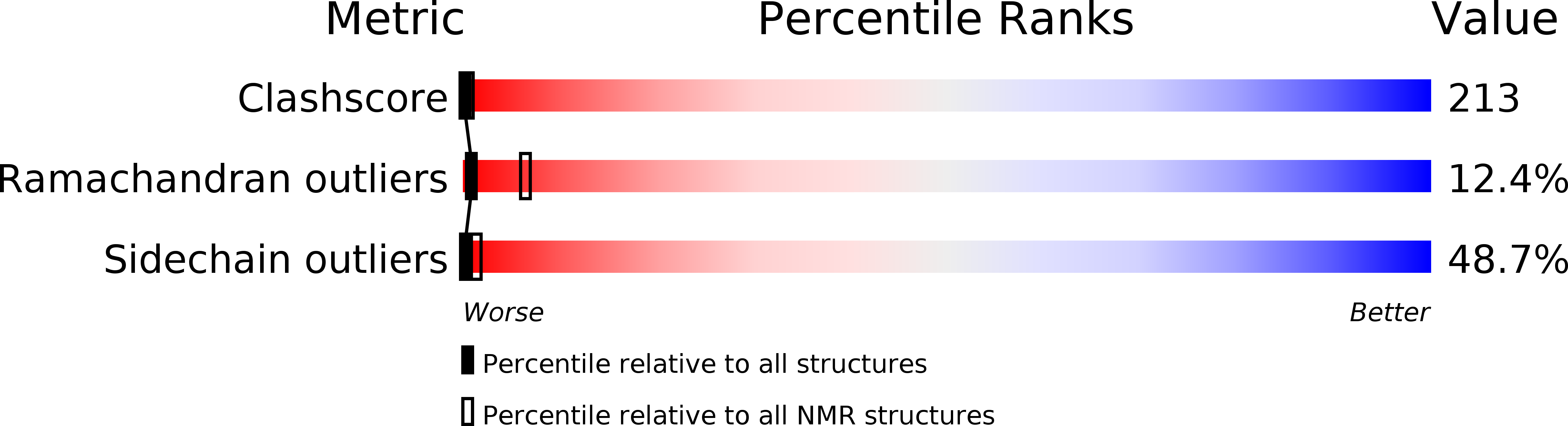Three-dimensional structure of the histidine-containing phosphocarrier protein (HPr) from Enterococcus faecalis in solution.
Maurer, T., Doker, R., Gorler, A., Hengstenberg, W., Kalbitzer, H.R.(2001) Eur J Biochem 268: 635-644
- PubMed: 11168402
- DOI: https://doi.org/10.1046/j.1432-1327.2001.01916.x
- Primary Citation of Related Structures:
1QFR - PubMed Abstract:
The histidine-containing phosphocarrier protein (HPr) transfers a phosphate group between components of the prokaryotic phosphoenolpyruvate-dependent phosphotransferase system (PTS), which is finally used to phosphorylate the carbohydrate transported by the PTS through the cell membrane. Recently it has also been found to act as an intermediate in the signaling cascade that regulates transcription of genes related to the carbohydrate-response system. Both functions involve phosphorylation/dephosphorylation reactions, but at different sites. Using multidimensional (1)H-NMR spectroscopy and angular space simulated annealing calculations, we determined the structure of HPr from Enterococcus faecalis in aqueous solution using 1469 distance and 44 angle constraints derived from homonuclear NMR data. It has a similar overall fold to that found in HPrs from other organisms. Four beta strands, A, B, C, D, encompassing residues 2-7, 32-37, 40-42 and 60-66, form an antiparallel beta sheet lying opposite the two antiparallel alpha helices, a and c (residues 16-26 and 70-83). A short alpha helix, b, from residues 47-53 is also observed. The pairwise root mean square displacement for the backbone heavy atoms of the mean of the 16 NMR structures to the crystal structure is 0.164 nm. In contrast with the crystalline state, in which a torsion angle strain in the active-center loop has been described [Jia, Z., Vandonselaar, M., Quail, J.W. & Delbaere, L.T.J. (1993) Nature (London) 361, 94-97], in the solution structure, the active-site His15 rests on top of helix a, and the phosphorylation site N(delta 1) of the histidine ring is oriented towards the surface, making it easily accessible to the solvent. Back calculation of the 2D NOESY NMR spectra from both the NMR and X-ray structures shows that the active-center structure derived by X-ray crystallography is not compatible with experimental data recorded in solution. The observed torsional strain must either be a crystallization artefact or represents a conformational state that exists only to a small extent in solution.
Organizational Affiliation:
Institut für Biophysik und Physikalische Biochemie, Universität Regensburg, Germany.