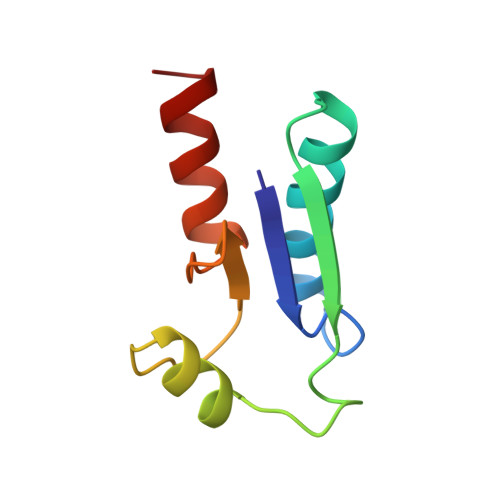
Binding specificity and mechanistic insight into glutaredoxin-catalyzed protein disulfide reduction.
Berardi, M.J., Bushweller, J.H.(1999) J Mol Biol 292: 151-161
- PubMed: 10493864
- DOI: https://doi.org/10.1006/jmbi.1999.3067
- Primary Citation of Related Structures:
1QFN - PubMed Abstract:
The reduction equivalents necessary for the ribonucleotide reductase (RNR)-catalyzed production of deoxyribonucleotides are provided by glutaredoxin (Grx) or thioredoxin (Trx). The initial location for transfer of reducing equivalents to RNR is located at the C terminus of the B1 subunit and involves the reduction of a disulfide between Cys754 and Cys759. We have used a 25-mer peptide corresponding to residues 737-761 of RNR B1 (C754-->S) to synthesize a stable mixed disulfide with Escherichia coli Grx-1 (C14-->S) resembling the structure of an intermediate in the reaction. The high-resolution solution structure of the mixed disulfide has been obtained by NMR with an RMSD of 0.56 A for all the backbone atoms of the protein and the well-defined portion of the peptide. The binding interactions responsible for specificity have been identified demonstrating the importance of electrostatic interactions in this system and providing a rationale for the specificity of the Grx-RNR interaction. The disulfide is buried in this complex, implying a solely intra-molecular mechanism of reduction in contrast to the previously determined structure of the glutathione complex where the disulfide was exposed; mutagenesis studies have shown the relevance of intermolecular reduction processes. Substantial conformational changes in the helices of the protein are associated with peptide binding which have significant mechanistic implications for protein disulfide reduction by glutaredoxins.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22906-0011, USA.