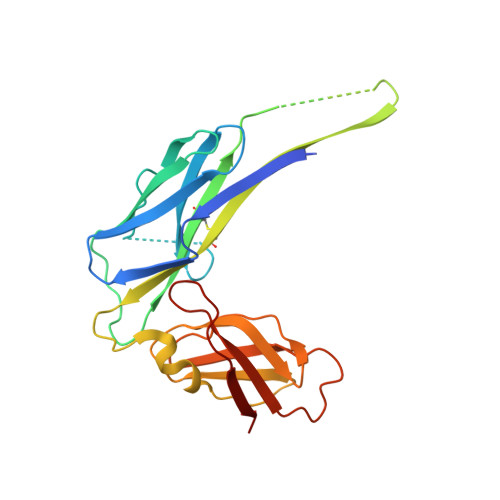
Structure and Biogenesis of the Capsular F1 Antigen from Yersinia pestis. Preserved Folding Energy Drives Fiber Formation
Zavialov, A.V., Berglund, J., Pudney, A.F., Fooks, L.J., Ibrahim, T.M., MacIntyre, S., Knight, S.D.(2003) Cell 113: 587-596
- PubMed: 12787500
- DOI: https://doi.org/10.1016/s0092-8674(03)00351-9
- Primary Citation of Related Structures:
1P5U, 1P5V - PubMed Abstract:
Most gram-negative pathogens express fibrous adhesive virulence organelles that mediate targeting to the sites of infection. The F1 capsular antigen from the plague pathogen Yersinia pestis consists of linear fibers of a single subunit (Caf1) and serves as a prototype for nonpilus organelles assembled via the chaperone/usher pathway. Genetic data together with high-resolution X-ray structures corresponding to snapshots of the assembly process reveal the structural basis of fiber formation. Comparison of chaperone bound Caf1 subunit with the subunit in the fiber reveals a novel type of conformational change involving the entire hydrophobic core of the protein. The observed conformational change suggests that the chaperone traps a high-energy folding intermediate of Caf1. A model is proposed in which release of the subunit allows folding to be completed, driving fiber formation.
Organizational Affiliation:
Department of Molecular Biosciences/Structural Biology, Uppsala Biomedical Center, Swedish University of Agricultural Sciences, Box 590, SE-751 24 Uppsala, Sweden.