NMR Solution Structure of Murine Ccl20/Mip3-A, a Chemiokine that Specifically Chemoattracs Immature Dendritic Cells and Lymphocytes Through its Highly Specific Interaction with the Beta-Chemokine Receptor Ccr6
Perez-Canadillas, J.M., Zaballos, A., Gutierrez, J., Varona, R., Roncal, F., Albar, J.P., Marquez, G., Bruix, M.(2001) J Biol Chem 276: 28372
- PubMed: 11373289
- DOI: https://doi.org/10.1074/jbc.M103121200
- Primary Citation of Related Structures:
1HA6 - PubMed Abstract:
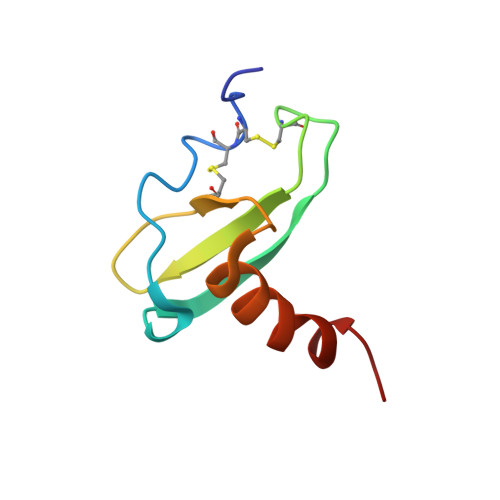
CCL20/MIP-3alpha is a beta-chemokine expressed in the thymus, skin, and intestinal epithelial cells that exclusively binds and activates the CCR6 receptor in both mice and humans. The strict receptor binding specificity of CCL20 is exceptional; other chemokines and their receptors bind promiscuously with multiple partners. Toward determining the structural basis for the selective receptor specificity of CCL20, we have determined its three-dimensional structure by 1H NMR spectroscopy. CCL20 exhibits the same monomeric structure previously described for other chemokines: a three-stranded beta-sheet and an overlying alpha-helix. The CCL20 receptor selectivity could arise from the rigid conformation of the N-terminal DCCL motif as well as the groove between the N-loop and the beta2-beta3 hairpin, which is significantly narrower in CCL20 than in other chemokines. Similar structural features are seen in human beta-defensin 2, a small nonchemokine polypeptide reported to selectively bind and activate CCR6, which stresses their importance for the specific binding of both CCL20 and beta-defensin 2 to CCR6. CCL20's structure will be useful to design tools aimed to modulate its important biological functions.
Organizational Affiliation:
Instituto de Estructura de la Materia, Consejo Superior de Investigaciones Cientificas, Serrano 119, 28006 Madrid, Spain.