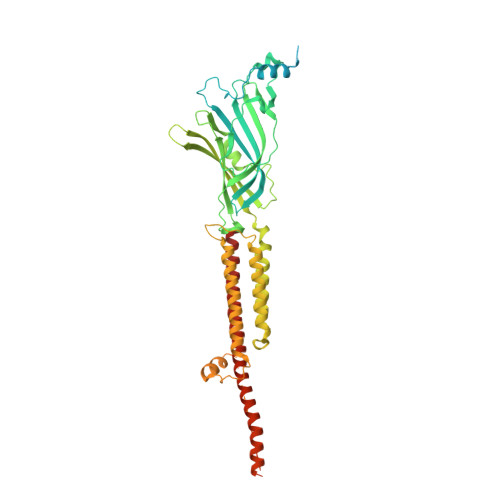
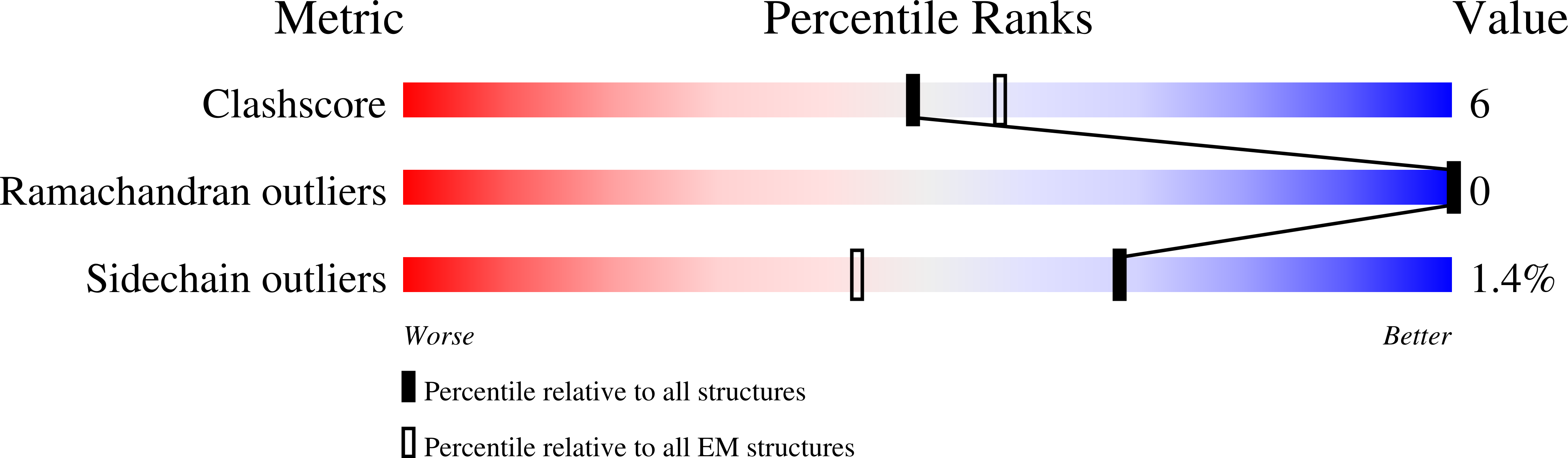Structural determinants for activity of the antidepressant vortioxetine at human and rodent 5-HT 3 receptors.
Lopez-Sanchez, U., Munro, L.J., Ladefoged, L.K., Pedersen, A.J., Brun, C.C., Lyngby, S.M., Baud, D., Juillan-Binard, C., Pedersen, M.G., Lummis, S.C.R., Bang-Andersen, B., Schiott, B., Chipot, C., Schoehn, G., Neyton, J., Dehez, F., Nury, H., Kristensen, A.S.(2024) Nat Struct Mol Biol
- PubMed: 38698207
- DOI: https://doi.org/10.1038/s41594-024-01282-x
- Primary Citation of Related Structures:
8AW2, 8AXD, 8BL8, 8BLA, 8BLB - PubMed Abstract:
Vortioxetine (VTX) is a recently approved antidepressant that targets a variety of serotonin receptors. Here, we investigate the drug's molecular mechanism of operation at the serotonin 5-HT 3 receptor (5-HT 3 R), which features two properties: VTX acts differently on rodent and human 5-HT 3 R, and VTX appears to suppress any subsequent response to agonists. Using a combination of cryo-EM, electrophysiology, voltage-clamp fluorometry and molecular dynamics, we show that VTX stabilizes a resting inhibited state of the mouse 5-HT 3 R and an agonist-bound-like state of human 5-HT 3 R, in line with the functional profile of the drug. We report four human 5-HT 3 R structures and show that the human receptor transmembrane domain is intrinsically fragile. We also explain the lack of recovery after VTX administration via a membrane partition mechanism.
Organizational Affiliation:
University Grenoble Alpes, CNRS, CEA, IBS, Grenoble, France.