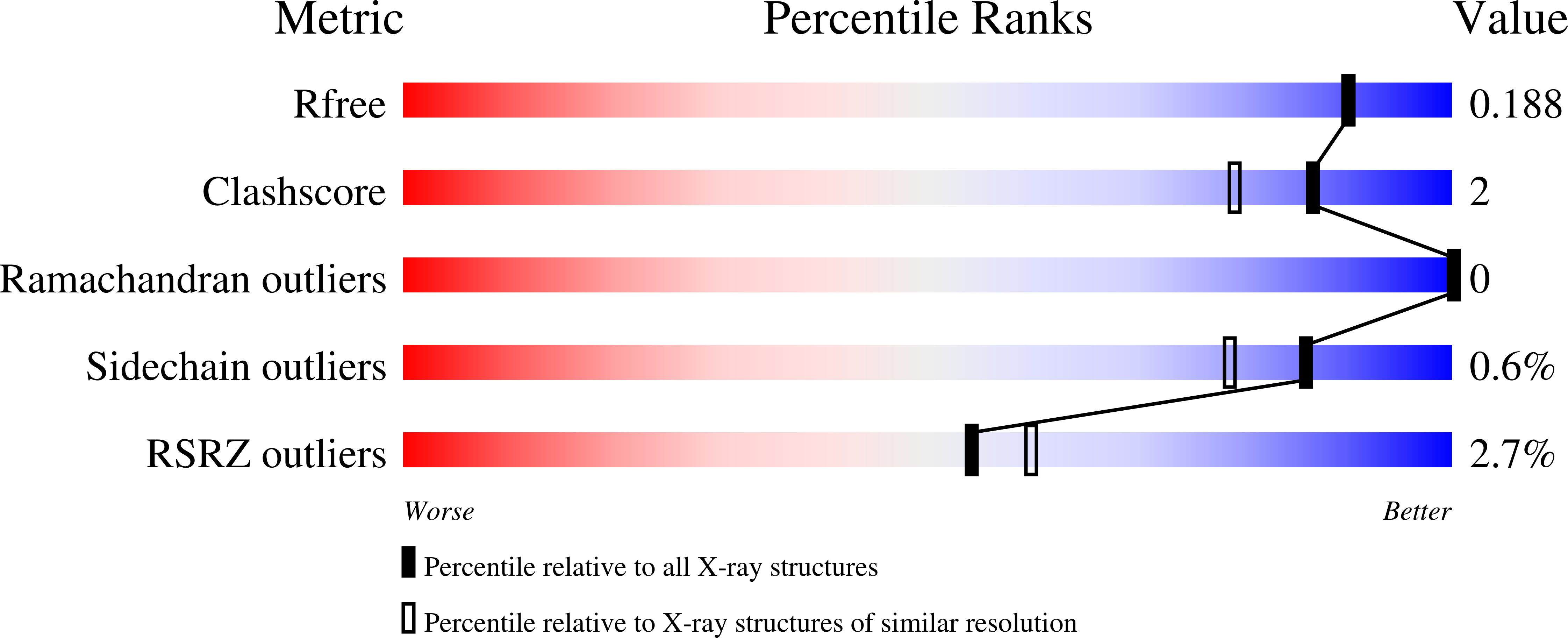
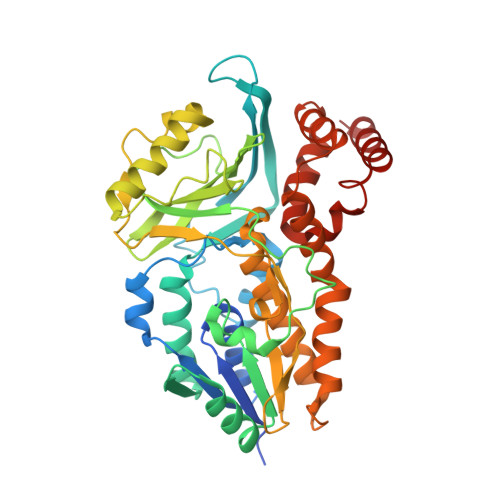
Structural and Mechanistic Studies on Substrate and Stereoselectivity of the Indole Monooxygenase VpIndA1: New Avenues for Biocatalytic Epoxidations and Sulfoxidations.
Kratky, J., Eggerichs, D., Heine, T., Hofmann, S., Sowa, P., Weisse, R.H., Tischler, D., Strater, N.(2023) Angew Chem Int Ed Engl 62: e202300657-e202300657
- PubMed: 36762980
- DOI: https://doi.org/10.1002/anie.202300657
- Primary Citation of Related Structures:
7Z4X, 7Z94, 7Z97, 7Z98, 7Z99, 7ZCA - PubMed Abstract:
Flavoprotein monooxygenases are a versatile group of enzymes for biocatalytic transformations. Among these, group E monooxygenases (GEMs) catalyze enantioselective epoxidation and sulfoxidation reactions. Here, we describe the crystal structure of an indole monooxygenase from the bacterium Variovorax paradoxus EPS, a GEM designated as VpIndA1. Complex structures with substrates reveal productive binding modes that, in conjunction with force-field calculations and rapid mixing kinetics, reveal the structural basis of substrate and stereoselectivity. Structure-based redesign of the substrate cavity yielded variants with new substrate selectivity (for sulfoxidation of benzyl phenyl sulfide) or with greatly enhanced stereoselectivity (from 35.1 % to 99.8 % ee for production of (1S,2R)-indene oxide). This first determination of the substrate binding mode of GEMs combined with structure-function relationships opens the door for structure-based design of these powerful biocatalysts.
Organizational Affiliation:
Institute of Bioanalytical Chemistry, Leipzig University, Deutscher Platz 5, 04103, Leipzig, Germany.