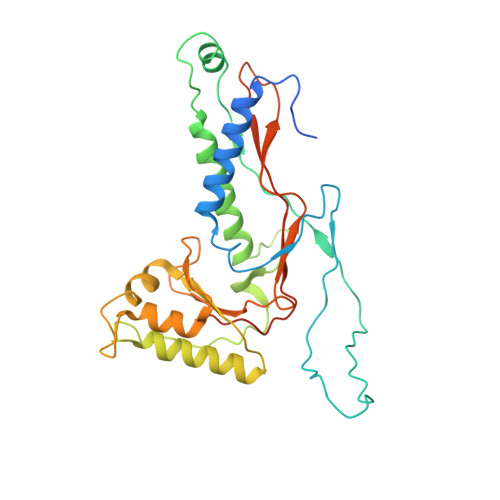
Structural characterization of the Myxococcus xanthus encapsulin and ferritin-like cargo system gives insight into its iron storage mechanism.
Eren, E., Wang, B., Winkler, D.C., Watts, N.R., Steven, A.C., Wingfield, P.T.(2022) Structure 30: 551-563.e4
- PubMed: 35150605
- DOI: https://doi.org/10.1016/j.str.2022.01.008
- Primary Citation of Related Structures:
7S20, 7S21, 7S2T, 7S4Q, 7S5C, 7S5K, 7S8T - PubMed Abstract:
Encapsulins are bacterial organelle-like cages involved in various aspects of metabolism, especially protection from oxidative stress. They can serve as vehicles for a wide range of medical applications. Encapsulin shell proteins are structurally similar to HK97 bacteriophage capsid protein and their function depends on the encapsulated cargos. The Myxococcus xanthus encapsulin system comprises EncA and three cargos: EncB, EncC, and EncD. EncB and EncC are similar to bacterial ferritins that can oxidize Fe +2 to less toxic Fe +3 . We analyzed EncA, EncB, and EncC by cryo-EM and X-ray crystallography. Cryo-EM shows that EncA cages can have T = 3 and T = 1 symmetry and that EncA T = 1 has a unique protomer arrangement. Also, we define EncB and EncC binding sites on EncA. X-ray crystallography of EncB and EncC reveals conformational changes at the ferroxidase center and additional metal binding sites, suggesting a mechanism for Fe oxidation and storage within the encapsulin shell.
Organizational Affiliation:
Protein Expression Laboratory, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD 20892, USA. Electronic address: elif.eren@nih.gov.