Structural basis and regulation of the reductive stress response.
Manford, A.G., Mena, E.L., Shih, K.Y., Gee, C.L., McMinimy, R., Martinez-Gonzalez, B., Sherriff, R., Lew, B., Zoltek, M., Rodriguez-Perez, F., Woldesenbet, M., Kuriyan, J., Rape, M.(2021) Cell 184: 5375-5390.e16
- PubMed: 34562363
- DOI: https://doi.org/10.1016/j.cell.2021.09.002
- Primary Citation of Related Structures:
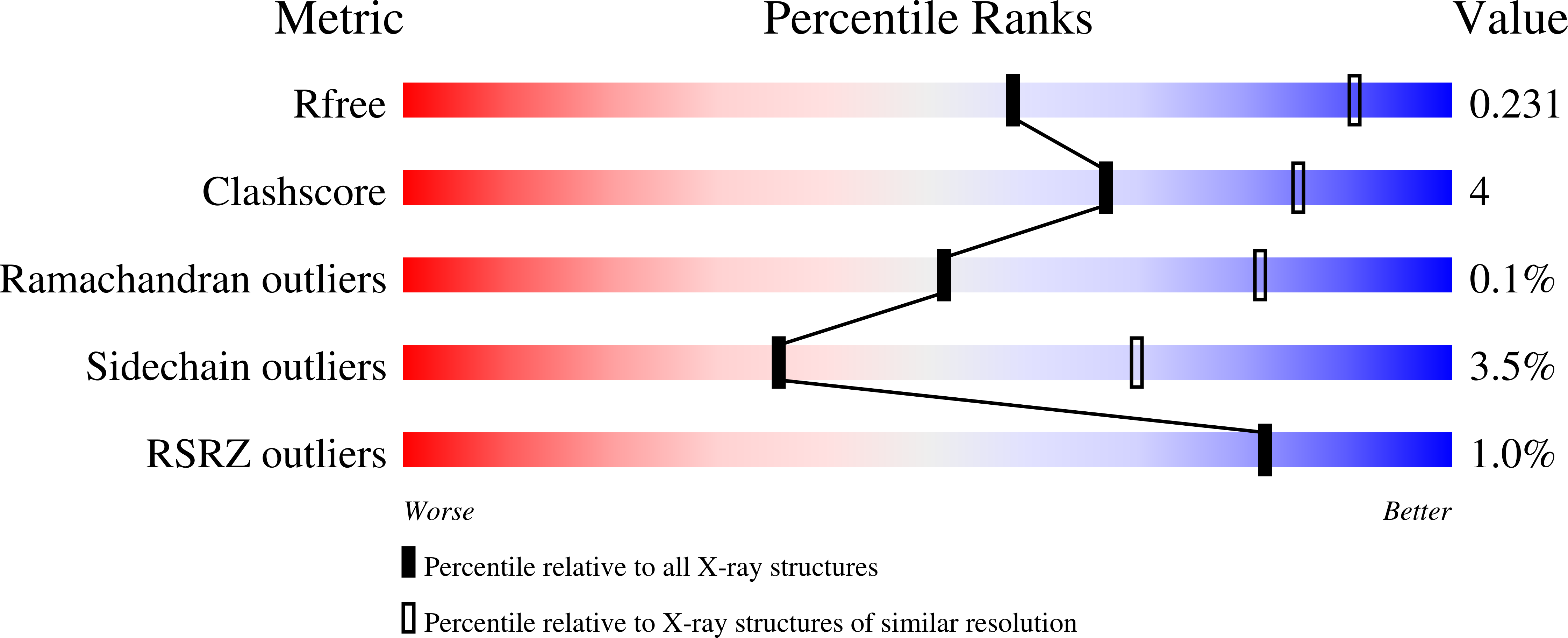
7ROY - PubMed Abstract:
Although oxidative phosphorylation is best known for producing ATP, it also yields reactive oxygen species (ROS) as invariant byproducts. Depletion of ROS below their physiological levels, a phenomenon known as reductive stress, impedes cellular signaling and has been linked to cancer, diabetes, and cardiomyopathy. Cells alleviate reductive stress by ubiquitylating and degrading the mitochondrial gatekeeper FNIP1, yet it is unknown how the responsible E3 ligase CUL2 FEM1B can bind its target based on redox state and how this is adjusted to changing cellular environments. Here, we show that CUL2 FEM1B relies on zinc as a molecular glue to selectively recruit reduced FNIP1 during reductive stress. FNIP1 ubiquitylation is gated by pseudosubstrate inhibitors of the BEX family, which prevent premature FNIP1 degradation to protect cells from unwarranted ROS accumulation. FEM1B gain-of-function mutation and BEX deletion elicit similar developmental syndromes, showing that the zinc-dependent reductive stress response must be tightly regulated to maintain cellular and organismal homeostasis.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA 94720, USA; Howard Hughes Medical Institute, University of California at Berkeley, Berkeley, CA 94720, USA.