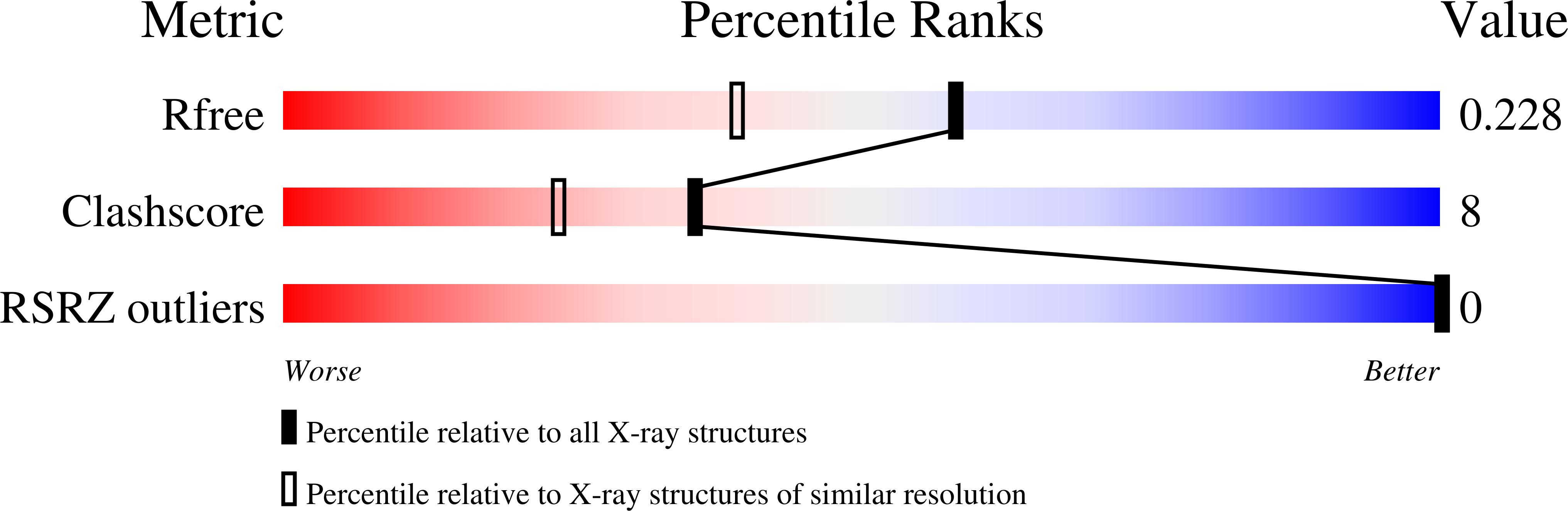RNA polymerase II trapped on a molecular treadmill: Structural basis of persistent transcriptional arrest by a minor groove DNA binder.
Oh, J., Jia, T., Xu, J., Chong, J., Dervan, P.B., Wang, D.(2022) Proc Natl Acad Sci U S A 119
- PubMed: 35022237
- DOI: https://doi.org/10.1073/pnas.2114065119
- Primary Citation of Related Structures:
7RIL, 7RIM, 7RIP, 7RIQ, 7RIW, 7RIX, 7RIY - PubMed Abstract:
Elongating RNA polymerase II (Pol II) can be paused or arrested by a variety of obstacles. These obstacles include DNA lesions, DNA-binding proteins, and small molecules. Hairpin pyrrole-imidazole (Py-Im) polyamides bind to the minor groove of DNA in a sequence-specific manner and induce strong transcriptional arrest. Remarkably, this Py-Im-induced Pol II transcriptional arrest is persistent and cannot be rescued by transcription factor TFIIS. In contrast, TFIIS can effectively rescue the transcriptional arrest induced by a nucleosome barrier. The structural basis of Py-Im-induced transcriptional arrest and why TFIIS cannot rescue this arrest remain elusive. Here we determined the X-ray crystal structures of four distinct Pol II elongation complexes (Pol II ECs) in complex with hairpin Py-Im polyamides as well as of the hairpin Py-Im polyamides-dsDNA complex. We observed that the Py-Im oligomer directly interacts with RNA Pol II residues, introduces compression of the downstream DNA duplex, prevents Pol II forward translocation, and induces Pol II backtracking. These results, together with biochemical studies, provide structural insight into the molecular mechanism by which Py-Im blocks transcription. Our structural study reveals why TFIIS fails to promote Pol II bypass of Py-Im-induced transcriptional arrest.
Organizational Affiliation:
Division of Pharmaceutical Sciences, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California San Diego, La Jolla, CA 92093.