Knotting terminal ends of mutant T1 lipase with disulfide bond improved structure rigidity and stability.
Hamdan, S.H., Maiangwa, J., Nezhad, N.G., Ali, M.S.M., Normi, Y.M., Shariff, F.M., Rahman, R.N.Z.R.A., Leow, T.C.(2023) Appl Microbiol Biotechnol 107: 1673-1686
- PubMed: 36752811
- DOI: https://doi.org/10.1007/s00253-023-12396-5
- Primary Citation of Related Structures:
7EY3 - PubMed Abstract:
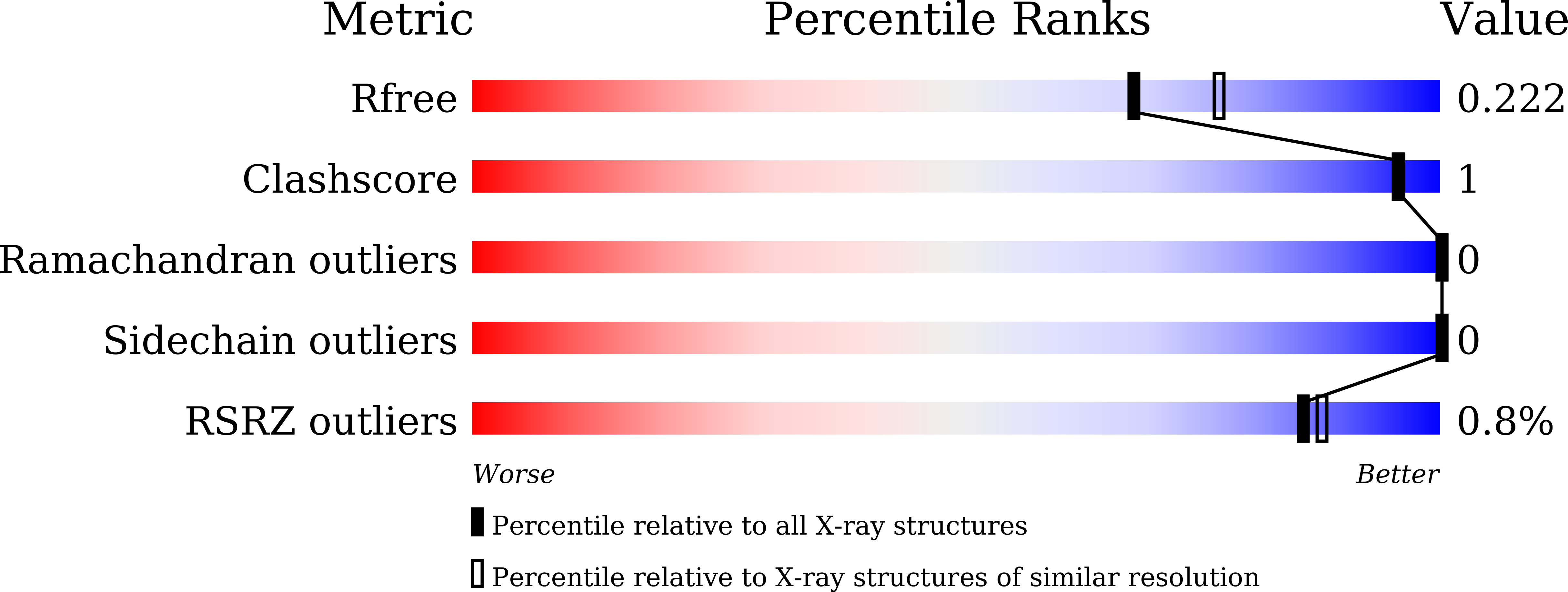
Lipase biocatalysts offer unique properties which are often impaired by low thermal and methanol stability. In this study, the rational design was employed to engineer a disulfide bond in the protein structure of Geobacillus zalihae T1 lipase in order to improve its stability. The selection of targeted disulfide bond sites was based on analysis of protein spatial configuration and change of Gibbs free energy. Two mutation points (S2C and A384C) were generated to rigidify the N-terminal and C-terminal regions of T1 lipase. The results showed the mutant 2DC lipase improved methanol stability from 35 to 40% (v/v) after 30 min of pre-incubation. Enhancement in thermostability for the mutant 2DC lipase at 70 °C and 75 °C showed higher half-life at 70 °C and 75 °C for 30 min and 52 min, respectively. The mutant 2DC lipase maintained the same optimum temperature (70 °C) as T1 lipase, while thermally induced unfolding showed the mutant maintained higher rigidity. The kcat/Km values demonstrated a relatively small difference between the T1 lipase (WT) and 2DC lipase (mutant). The kcat/Km (s -1 mM -1 ) of the T1 and 2DC showed values of 13,043 ± 224 and 13,047 ± 312, respectively. X-ray diffraction of 2DC lipase crystal structure with a resolution of 2.04 Å revealed that the introduced single disulfide bond did not lower initial structural interactions within the residues. Enhanced methanol and thermal stability are suggested to be strongly related to the newly disulfide bridge formation and the enhanced compactness and rigidity of the mutant structure. KEY POINTS: • Protein engineering via rational design revealed relative improved enzymatic performance. • The presence of disulfide bond impacts on the rigidity and structural function of proteins. • X-ray crystallography reveals structural changes accompanying protein modification.
Organizational Affiliation:
Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Science, Universiti Putra Malaysia Serdang, UPM Serdang, 43400, Selangor, Malaysia.