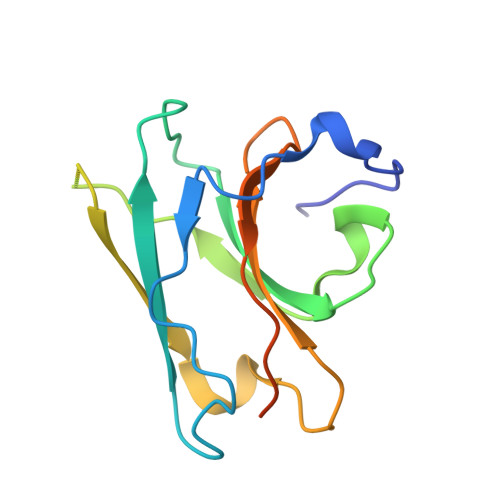
Structural and biochemical advances on the recruitment of the autophagy-initiating ULK and TBK1 complexes by autophagy receptor NDP52.
Fu, T., Zhang, M., Zhou, Z., Wu, P., Peng, C., Wang, Y., Gong, X., Li, Y., Wang, Y., Xu, X., Li, M., Shen, L., Pan, L.(2021) Sci Adv 7
- PubMed: 34389544
- DOI: https://doi.org/10.1126/sciadv.abi6582
- Primary Citation of Related Structures:
7EA2, 7EA7, 7EAA - PubMed Abstract:
The recruitment of Unc-51-like kinase and TANK-binding kinase 1 complexes is essential for Nuclear dot protein 52-mediated selective autophagy and relies on the specific association of NDP52, RB1-inducible coiled-coil protein 1, and Nak-associated protein 1 (5-azacytidine-induced protein 2, AZI2). However, the underlying molecular mechanism remains elusive. Here, we find that except for the NDP52 SKIP carboxyl homology (SKICH)/RB1CC1 coiled-coil interaction, the LC3-interacting region of NDP52 can directly interact with the RB1CC1 Claw domain, as that of NAP1 FIP200-binding region (FIR). The determined crystal structures of NDP52 SKICH/RB1CC1 complex, NAP1 FIR/RB1CC1 complex, and the related NAP1 FIR/Gamma-aminobutyric acid receptor-associated protein complex not only elucidate the molecular bases underpinning the interactions of RB1CC1 with NDP52 and NAP1 but also reveal that RB1CC1 Claw and Autophagy-related protein 8 family proteins are competitive in binding to NAP1 and NDP52. Overall, our findings provide mechanistic insights into the interactions of NDP52, NAP1 with RB1CC1 and ATG8 family proteins.
Organizational Affiliation:
State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China.