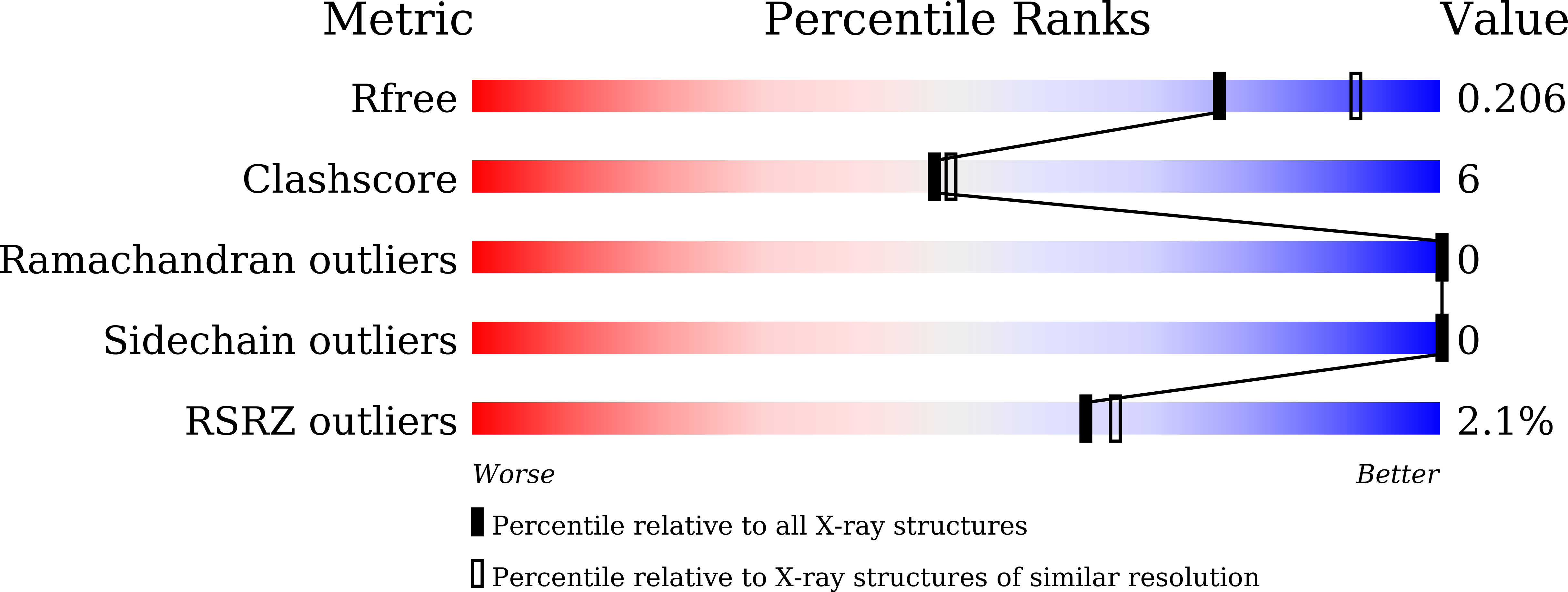
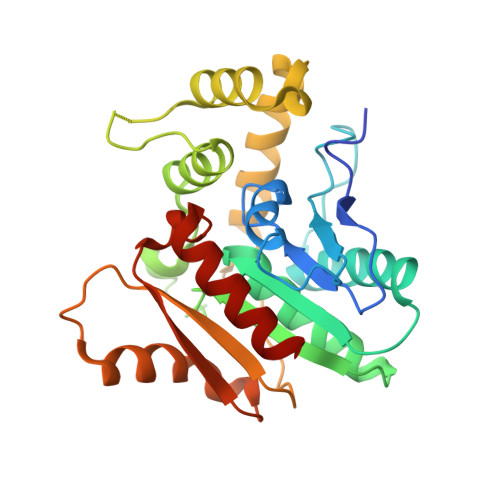
Functional Characterization and Crystal Structure of the Bifunctional Thioesterase Catalyzing Epimerization and Cyclization in Skyllamycin Biosynthesis
Yu, J., Juan, S., Chi, C., Liu, T., Geng, T., Cai, Z., Dong, W., Shi, C., Ma, X., Zhang, Z., Ma, X., Xing, B., Jin, H., Zhang, L., Dong, S., Yang, D., Ma, M.(2021) ACS Catal 11: 11733-11741