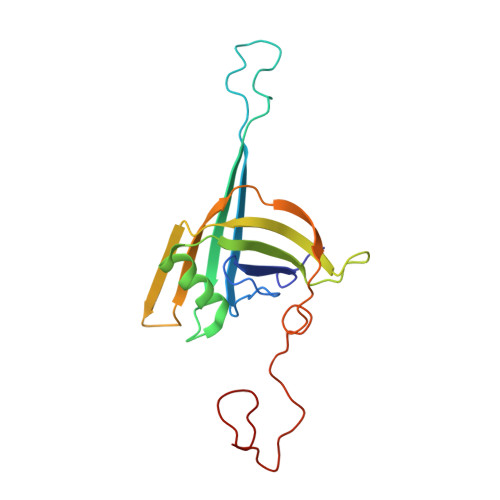
Architecture of the flexible tail tube of bacteriophage SPP1.
Zinke, M., Sachowsky, K.A.A., Oster, C., Zinn-Justin, S., Ravelli, R., Schroder, G.F., Habeck, M., Lange, A.(2020) Nat Commun 11: 5759-5759
- PubMed: 33188213
- DOI: https://doi.org/10.1038/s41467-020-19611-1
- Primary Citation of Related Structures:
6YEG, 6YQ5 - PubMed Abstract:
Bacteriophage SPP1 is a double-stranded DNA virus of the Siphoviridae family that infects the bacterium Bacillus subtilis. This family of phages features a long, flexible, non-contractile tail that has been difficult to characterize structurally. Here, we present the atomic structure of the tail tube of phage SPP1. Our hybrid structure is based on the integration of structural restraints from solid-state nuclear magnetic resonance (NMR) and a density map from cryo-EM. We show that the tail tube protein gp17.1 organizes into hexameric rings that are stacked by flexible linker domains and, thus, form a hollow flexible tube with a negatively charged lumen suitable for the transport of DNA. Additionally, we assess the dynamics of the system by combining relaxation measurements with variances in density maps.
Organizational Affiliation:
Department of Molecular Biophysics, Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), Berlin, Germany.